| Content: | 3 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 2 years under recommended storage conditions |
| Substrate For (Enzyme): | endo-1,4-β-Xylanase |
| Assay Format: | Spectrophotometer, Petri-dish (Qualitative) |
| Detection Method: | Absorbance |
| Wavelength (nm): | 590 |
| Reproducibility (%): | ~ 7% |
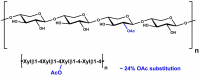
High purity dyed, soluble Azo-Xylan (Birchwood) for the measurement of enzyme activity, for research, biochemical enzyme assays and analytical testing applications.
Substrate for the specific assay of endo-1,4-β-D-xylanase.
Please note the video above shows the protocol for assay of endo-cellulase using Azo-CM cellulose. The procedure for the assay of endo-1,4-β-xylanase using Azo-Xylan (Birchwood) (Powder) is equivalent to this.
View other related products for more enzyme substrates.
Mangan, D., Cornaggia, C., Liadova, A., McCormack, N., Ivory, R., McKie, V. A., Ormerod, A. & McCleary, D. V. (2017). Carbohydrate Research, 445, 14-22.
endo-1,4-β-Xylanase (EC 3.2.1.8) is employed across a broad range of industries including animal feed, brewing, baking, biofuels, detergents and pulp (paper). Despite its importance, a rapid, reliable, reproducible, automatable assay for this enzyme that is based on the use of a chemically defined substrate has not been described to date. Reported herein is a new enzyme coupled assay procedure, termed the XylX6 assay, that employs a novel substrate, namely 4,6-O-(3-ketobutylidene)-4-nitrophenyl-β-45-O-glucosyl-xylopentaoside. The development of the substrate and associated assay is discussed here and the relationship between the activity values obtained with the XylX6 assay versus traditional reducing sugar assays and its specificity and reproducibility were thoroughly investigated.
Hide AbstractImprovement of β‐Xylosidase and Endoxylanase Activities in Talaromyces amestolkiae by Genetic Manipulation of the Transcriptional Activator XlnR.
Pozo‐Rodríguez, A., Peñalva, M. Á., Barriuso, J., Espeso, E. A. & Martínez, M. J. (2025). Microbial Biotechnology, 18(5), e70166.
The ascomycete Talaromyces amestolkiae is a promising source of glycosyl hydrolases for hemicellulose degradation, as it contains a considerably higher number of genes encoding these enzymes than other fungi exploited for plant biomass valorisation. The development of genetic engineering tools could further improve its biotechnological potential. We report here a transformation system for T. amestolkiae based on pyrimidine auxotrophy complementation, which was used to successfully introduce both integrative and autonomously replicating plasmids. Then, we applied this tool to force the expression of the transcriptional activator XlnR, generating an engineered strain with enhanced β‐xylosidase (1.4‐fold) and endoxylanase (2.0‐fold) activities compared to the wild‐type cultured on xylan. Markedly larger improvements were obtained after introducing Ala788Val or Val785Phe substitutions in XlnR, achieving 3.3‐fold and 3.9‐fold increases in β‐xylosidase and endoxylanase activities, respectively, in the case of XlnRV785F. This recombinant strain also displays a partial deregulation of the hemicellulolytic system when cultivated on glucose and glycerol (a low‐cost and renewable substrate), yielding notably higher production of β‐xylosidases (16.9‐fold and 13.8‐fold) and endoxylanases (31.9‐fold and 22.7‐fold) than the wild‐type. Increased efficiencies of XlnRV785F enzymatic crudes in xylan saccharifications showed the potential of XlnR engineering to develop robust T. amestolkiae strains for the valorisation of hemicellulosic residues.
Hide AbstractStructural and functional insights into extreme thermal stability and activity of two GH 12 domains of a multidomain glycosidase from a hyperthermophilic euryarchaeon.
Zayulina, K. S., Frolov, E. N., Stracke, C., Klyukina, A. A., Khusnutdinova, A. N., Stogios, P., Skarina, T., Yakunin, A., Golyshin, P. N., Siebers, B., Shugaeva, T. E. & Kublanov, I. V. (2025). The FEBS Journal, 70095.
Bacteria and fungi are well known for efficient degradation of plant polysaccharides thanks to various enzymes involved in plant cell wall decomposition. However, little is known about the role of archaea in this process or the repertoire and features of their polysaccharide-degrading enzymes. In our previous work, we discovered an archaeal multidomain glycosidase (MDG) composed of three catalytic domains (GH5 and two GH12) and two cellulose-binding modules (CBM2). The recombinant MDG and individual GH5 catalytic domain were active against cellulose and a number of other polysaccharides at a wide range of temperatures, with optimum temperatures (Topt) of 60°C and 80°C, respectively. The present study was focused on the characterization of two GH12 domains of the MDG. Purified recombinant TMDG_GH12-1 and TMDG_GH12-2 proteins were active as individual enzymes but exhibited distinct catalytic properties. Both enzymes were thermostable and active at extremely high temperatures: TMDG_GH12-1 was active at 40-130°C (Topt 100°C), and its half-life (t½) at 100°C was 42 h, which makes it one of the most thermostable glycosidases known so far, whereas TMDG_GH12-2 was active at 50-100°C (Topt 90°C) with t½ at 100°C being 30 min. Phylogenetic and structural analysis of both TMDG_GH12 proteins together with molecular docking and site-directed mutagenesis suggested that the presence of two disulfide bridges and the W → Q mutation in the active site contribute to the exceptional thermostability of TMDG_GH12-1. Further structural and mutational studies of the TMDG_GH12-1 domain will help to gain a better understanding of the molecular mechanisms of its extraordinary thermostability and substrate specificity.
Hide AbstractIndependent metabolism of oligosaccharides is the keystone of synchronous utilization of cellulose and hemicellulose in Myceliophthora.
Liu, J., Chen, M., Gu, S., Fan, R., Zhao, Z., Sun, W., Yao, Y., Li, J. & Tian, C. (2024). PNAS nexus, 3(2), pgae053.
The effective utilization of cellulose and hemicellulose, the main components of plant biomass, is a key technical obstacle that needs to be overcome for the economic viability of lignocellulosic biorefineries. Here, we firstly demonstrated that the thermophilic cellulolytic fungus Myceliophthora thermophila can simultaneously utilize cellulose and hemicellulose, as evidenced by the independent uptake and intracellular metabolism of cellodextrin and xylodextrin. When plant biomass serviced as carbon source, we detected the cellodextrin and xylodextrin both in cells and in the culture medium, as well as high enzyme activities related to extracellular oligosaccharide formation and intracellular oligosaccharide hydrolysis. Sugar consumption assay revealed that in contrast to inhibitory effect of glucose on xylose and cellodextrin/xylodextrin consumption in mixed-carbon media, cellodextrin and xylodextrin were synchronously utilized in this fungus. Transcriptomic analysis also indicated simultaneous induction of the genes involved in cellodextrin and xylodextrin metabolic pathway, suggesting carbon catabolite repression (CCR) is triggered by extracellular glucose and can be eliminated by the intracellular hydrolysis and metabolism of oligosaccharides. The xylodextrin transporter MtCDT-2 was observed to preferentially transport xylobiose and tolerate high cellobiose concentrations, which helps to bypass the inhibition of xylobiose uptake. Furthermore, the expression of cellulase and hemicellulase genes was independently induced by their corresponding inducers, which enabled this strain to synchronously utilize cellulose and hemicellulose. Taken together, the data presented herein will further elucidate the degradation of plant biomass by fungi, with implications for the development of consolidated bioprocessing-based lignocellulosic biorefinery.
Hide AbstractProduction Of Cellulosic Enzymes By Aspergillus Niger And Hydrolysis Of Cellulosic Materials.
Bakare, V., Abdulsalami, M. S., Ndibe, T. O., Ejuama, C. K. & Effiong, T. (2022). Global Journal of Pure and Applied Sciences, 28(2), 121-129.
Microorganisms such as fungi can fragment carbon compounds by hydrolytic enzymes. The filamentous fungus, Aspergillus niger is now mostly considered because of its ubiquitous nature, non-fastidious nutritional requirements and it is classified generally as safe. This study was aimed at the production of cellulosic enzymes by A. niger and hydrolytic degradation of cellulosic materials by these enzymes. Standard methods were employed in soil samples collection, isolation of A. niger from the soils and their screening for enzyme production. Results showed that the A. niger isolates exhibited considerable activities of degrading and hydrolyzing cellulose in the agar media. The highest FPase, cellulase and xylanase activities were obtained from white saw dust with concentrations of 0.4059 U/ml, 0.7695U/ml and 1.3488 U/ml respectively. Also, results showed high enzyme activity at pH 6 (0.52U/ml) and temperature of 30ºC (0.72U/ml). Acid hydrolysis of the cellulosic substrates resulted to the release of 6.5% total sugar from white sawdust. The findings of this study revealed that the enzymes produced by A. niger hydrolyzed cellulosic materials but acid is more efficient than the enzymes in the hydrolysis and release of total sugar from cellulosic materials. This study recommends that cellulolytic enzymes used in the industries should be produced locally using filamentous fungus such as Aspergillus niger and cellulosic materials as carbon source.
Hide AbstractPACER: a novel 3D plant cell wall model for the analysis of non-catalytic and enzymatic responses.
Monschein, M., Jurak, E., Paasela, T., Koitto, T., Lambauer, V., Pavicic, M., Enjalbert, T., Dumon, C. & Master, E. R. (2022). Biotechnology for Biofuels and Bioproducts, 15(1), 1-11.
Background: Substrate accessibility remains a key limitation to the efficient enzymatic deconstruction of lignocellulosic biomass. Limited substrate accessibility is often addressed by increasing enzyme loading, which increases process and product costs. Alternatively, considerable efforts are underway world-wide to identify amorphogenesis-inducing proteins and protein domains that increase the accessibility of carbohydrate-active enzymes to targeted lignocellulose components. Results: We established a three-dimensional assay, PACER (plant cell wall model for the analysis of non-catalytic and enzymatic responses), that enables analysis of enzyme migration through defined lignocellulose composites. A cellulose/azo-xylan composite was made to demonstrate the PACER concept and then used to test the migration and activity of multiple xylanolytic enzymes. In addition to non-catalytic domains of xylanases, the potential of loosenin-like proteins to boost xylanase migration through cellulose/azo-xylan composites was observed. Conclusions: The PACER assay is inexpensive and parallelizable, suitable for screening proteins for ability to increase enzyme accessibility to lignocellulose substrates. Using the PACER assay, we visualized the impact of xylan-binding modules and loosenin-like proteins on xylanase mobility and access to targeted substrates. Given the flexibility to use different composite materials, the PACER assay presents a versatile platform to study impacts of lignocellulose components on enzyme access to targeted substrates.
Hide AbstractDevelopment of an Efficient C-to-T Base-Editing System and Its Application to Cellulase Transcription Factor Precise Engineering in Thermophilic Fungus Myceliophthora thermophila.
Zhang, C., Li, N., Rao, L., Li, J., Liu, Q. & Tian, C. (2022). Microbiology Spectrum, e02321-21.
Myceliophthora thermophila is a thermophilic fungus with great potential in biorefineries and biotechnology. The base editor is an upgraded version of the clustered regularly interspaced short palindromic repeats (CRISPR)-dependent genome-editing tool that introduces precise point mutations without causing DNA double-strand breaks (DSBs) and has been used in various organisms but rarely in filamentous fungi, especially thermophilic filamentous fungi. Here, for the first time, we constructed three cytosine base editors (CBEs) in M. thermophila, namely, evolved apolipoprotein B mRNA-editing enzyme catalytic subunit 1 (APOBEC1) cytosine base editor 4 max (Mtevo-BE4max), bacteriophage Mu Gam protein cytosine base editor 4 max (MtGAM-BE4max), and evolved CDA1 deaminase cytosine base editor (Mtevo-CDA1), and efficiently inactivated genes by precisely converting three codons (CAA, CAG, and CGA) into stop codons without DSB formation. The Mtevo-CDA1 editor with up to 92.6% editing efficiency is a more suitable tool for cytosine base editing in thermophilic fungi. To investigate the function of each motif of the cellulase transcription factor M. thermophila CLR-2 (MtCLR-2), we used the Mtevo-CDA1 editor. The fungal-specific motif of MtCLR-2 was found to be strongly involved in cellulase secretion, conidium formation, hyphal branching, and colony formation. Mutation of the fungus-specific motif caused significant defects in these characteristics. Thus, we developed an efficient thermophilic fungus-compatible base-editing system that could also be used for genetic engineering in other relevant filamentous fungi.
Hide AbstractThe chimeric GaaR-XlnR transcription factor induces pectinolytic activities in the presence of D-xylose in Aspergillus niger.
Kun, R. S., Garrigues, S., Di Falco, M., Tsang, A. & de Vries, R. P. (2021). Applied Microbiology and Biotechnology, 105(13), 5553-5564.
Aspergillus niger is a filamentous fungus well known for its ability to produce a wide variety of pectinolytic enzymes, which have many applications in the industry. The transcriptional activator GaaR is induced by 2-keto-3-deoxy-L-galactonate, a compound derived from D-galacturonic acid, and plays a major role in the regulation of pectinolytic genes. The requirement for inducer molecules can be a limiting factor for the production of enzymes. Therefore, the generation of chimeric transcription factors able to activate the expression of pectinolytic genes by using underutilized agricultural residues would be highly valuable for industrial applications. In this study, we used the CRISPR/Cas9 system to generate three chimeric GaaR-XlnR transcription factors expressed by the xlnR promoter by swapping the N-terminal region of the xylanolytic regulator XlnR to that of the GaaR in A. niger. As a test case, we constructed a PpgaX-hph reporter strain to evaluate the alteration of transcription factor specificity in the chimeric mutants. Our results showed that the chimeric GaaR-XlnR transcription factor was induced in the presence of D-xylose. Additionally, we generated a constitutively active GaaR-XlnR V756F version of the most efficient chimeric transcription factor to better assess its activity. Proteomics analysis confirmed the production of several pectinolytic enzymes by ΔgaaR mutants carrying the chimeric transcription factor. This correlates with the improved release of D-galacturonic acid from pectin by the GaaR-XlnR V756F mutant, as well as by the increased L-arabinose release from the pectin side chains by both chimeric mutants under inducing condition, which is required for efficient degradation of pectin.
Hide AbstractEffect of ammonia fiber expansion-treated wheat straw and a recombinant fibrolytic enzyme on rumen microbiota and fermentation parameters, total tract digestibility, and performance of lambs.
Ribeiro, G. O., Gruninger, R. J., Jones, D. R., Beauchemin, K. A., Yang, W. Z., Wang, Y., Abbott, D. W., Tsang, A. & McAllister, T. A. (2020). Journal of Animal Science, 98(5), skaa116.
The objective of this study was to evaluate the effect of ammonia fiber expansion (AFEX)-treated wheat straw pellets and a recombinant fibrolytic enzyme on the rumen microbiome, rumen fermentation parameters, total tract diet digestibility, and performance of lambs. Eight rumen cannulated wethers and 60 lambs (n = 15 per diet, 8 rams and 7 ewes) were used in a replicated 4 × 4 Latin square design digestibility study and a complete randomized growth performance study, respectively. Four treatment diets were arranged in a 2 × 2 factorial structure with AFEX wheat straw (0% or 30% AFEX straw pellets on a dietary DM basis replacing alfalfa hay pellets) and fibrolytic enzyme (with or without XYL10C, a β-1,4-xylanase, from Aspergillus niger) as main factors. Enzyme was applied at 100 mg/kg of diet DM, 22 h before feeding. Rumen bacteria diversity Pielou evenness decreased (P = 0.05) with AFEX compared with the control diet and increased (P < 0.01) with enzyme. Enzyme increased (P ≤ 0.02) the relative abundancies of Prevotellaceae UCG-004, Christensenellaceae R-7 group, Saccharofermentans, and uncultured Kiritimatiellaeota. Total protozoa counts were greater (P ≤ 0.04) in the rumen of lambs fed AFEX compared with control, with enzyme reducing (P ≤ 0.05) protozoa counts for both diets. Digestibility of DM did not differ (P > 0.10) among diets, but digestibility of CP was reduced (P = 0.001), and digestibility of NDF and ADF increased (P < 0.05) as AFEX replaced alfalfa. Compared with control, AFEX promoted greater DMI (P = 0.003) and improved ADG up to 42 d on feed (P = 0.03), but not (P = 0.51) over the full ~94-d experiment. Consequently, overall G:F was reduced (P = 0.04) for AFEX when compared with control (0.188 vs. 0.199), but days on feed were lower (P = 0.04) for AFEX (97 vs. 91 d). Enzyme improved DMI of AFEX up to day 70 (P = 0.01), but did not affect DMI of the control diet. Enzyme addition improved ADG of lambs fed both diets in the first 28 d (P = 0.02), but not over the entire feeding period (P ≥ 10). As a result, G:F was improved with enzyme for the first 28 d (P = 0.04), but not overall (P = 0.45). This study shows that AFEX-treated wheat straw can replace alfalfa hay with no loss in lamb growth performance. Additionally, the enzyme XYL10C altered the rumen microbiome and improved G:F in the first month of the feeding.
Hide AbstractInnovative microscale workflow from fungi cultures to Cell Wall‐Degrading Enzyme screening.
Raulo, R., Heuson, E., Siah, A., Phalip, V. & Froidevaux, R. (2019). Microbial Biotechnology, 12(6), 1286-1292.
This study aimed at developing a complete miniaturized high‐throughput screening workflow for the evaluation of the Cell Wall‐Degrading Enzyme (CWDE) activities produced by any fungal strain directly cultivated on raw feedstock in a submerged manner. In this study, wheat straw was selected as model substrate as it represents an important carbon source but yet poorly valorised to yield high added value products. Fungi were grown in a microbioreactor in a high‐throughput (HT) way to replace the fastidious shaking flask cultivations. Both approaches were compared in order to validate our new methodology. The range of CWDE activities produced from the cultures was assayed using AZO‐died and pNP‐linked substrates in an SBS plate format using a Biomek FXp pipetting platform. As highlighted in this study, it was shown that the CWDE activities gathered from the microbioreactor cultivations were similar or higher to those obtained from shake flasks cultures, with a lower standard deviation on the measured values, making this new method much faster than the traditional one and suitable for HT CWDE production thanks to its pipetting platform compatibility. Also, the results showed that the enzymatic activities measured were the same when doing the assay manually or using the automated method.
Hide Abstract