| Content: | 10 g or 50 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 2 years under recommended storage conditions |
| CAS Number: | 9014-63-5 |
| Source: | Beechwood |
| Purity: | > 95% |
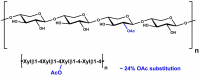
| Monosaccharides (%): | Xylose: Glucuronic Acid: Other sugars = 84: 10.3: 5.7 |
| Main Chain Glycosidic Linkage: | β-1,4 and α-1,2 |
| Substrate For (Enzyme): | endo-1,4-β-Xylanase |
Highly purified xylan from beechwood for use in research, biochemical enzyme assays and analytical testing applications.
Suitable as a replacement for birchwood xylan as a substrate for β-xylanase in DNSA reducing sugar assay.
Data booklets for each pack size are located in the Documents tab.
Mangan, D., Cornaggia, C., Liadova, A., McCormack, N., Ivory, R., McKie, V. A., Ormerod, A. & McCleary, D. V. (2017). Carbohydrate Research, 445, 14-22.
endo-1,4-β-Xylanase (EC 3.2.1.8) is employed across a broad range of industries including animal feed, brewing, baking, biofuels, detergents and pulp (paper). Despite its importance, a rapid, reliable, reproducible, automatable assay for this enzyme that is based on the use of a chemically defined substrate has not been described to date. Reported herein is a new enzyme coupled assay procedure, termed the XylX6 assay, that employs a novel substrate, namely 4,6-O-(3-ketobutylidene)-4-nitrophenyl-β-45-O-glucosyl-xylopentaoside. The development of the substrate and associated assay is discussed here and the relationship between the activity values obtained with the XylX6 assay versus traditional reducing sugar assays and its specificity and reproducibility were thoroughly investigated.
Hide AbstractMcCleary, B. V. & McGeough, P. (2015). Appl. Biochem. Biotechnol., 177(5), 1152-1163.
The most commonly used method for the measurement of the level of endo-xylanase in commercial enzyme preparations is the 3,5-dinitrosalicylic acid (DNS) reducing sugar method with birchwood xylan as substrate. It is well known that with the DNS method, much higher enzyme activity values are obtained than with the Nelson-Somogyi (NS) reducing sugar method. In this paper, we have compared the DNS and NS reducing sugar assays using a range of xylan-type substrates and accurately compared the molar response factors for xylose and a range of xylo-oligosaccharides. Purified beechwood xylan or wheat arabinoxylan is shown to be a suitable replacement for birchwood xylan which is no longer commercially available, and it is clearly demonstrated that the DNS method grossly overestimates endo-xylanase activity. Unlike the DNS assay, the NS assay gave the equivalent colour response with equimolar amounts of xylose, xylobiose, xylotriose and xylotetraose demonstrating that it accurately measures the quantity of glycosidic bonds cleaved by the endo-xylanase. The authors strongly recommend cessation of the use of the DNS assay for measurement of endo-xylanase due to the fact that the values obtained are grossly overestimated due to secondary reactions in colour development.
Hide AbstractImprovement of β‐Xylosidase and Endoxylanase Activities in Talaromyces amestolkiae by Genetic Manipulation of the Transcriptional Activator XlnR.
Pozo‐Rodríguez, A., Peñalva, M. Á., Barriuso, J., Espeso, E. A. & Martínez, M. J. (2025). Microbial Biotechnology, 18(5), e70166.
The ascomycete Talaromyces amestolkiae is a promising source of glycosyl hydrolases for hemicellulose degradation, as it contains a considerably higher number of genes encoding these enzymes than other fungi exploited for plant biomass valorisation. The development of genetic engineering tools could further improve its biotechnological potential. We report here a transformation system for T. amestolkiae based on pyrimidine auxotrophy complementation, which was used to successfully introduce both integrative and autonomously replicating plasmids. Then, we applied this tool to force the expression of the transcriptional activator XlnR, generating an engineered strain with enhanced β‐xylosidase (1.4‐fold) and endoxylanase (2.0‐fold) activities compared to the wild‐type cultured on xylan. Markedly larger improvements were obtained after introducing Ala788Val or Val785Phe substitutions in XlnR, achieving 3.3‐fold and 3.9‐fold increases in β‐xylosidase and endoxylanase activities, respectively, in the case of XlnRV785F. This recombinant strain also displays a partial deregulation of the hemicellulolytic system when cultivated on glucose and glycerol (a low‐cost and renewable substrate), yielding notably higher production of β‐xylosidases (16.9‐fold and 13.8‐fold) and endoxylanases (31.9‐fold and 22.7‐fold) than the wild‐type. Increased efficiencies of XlnRV785F enzymatic crudes in xylan saccharifications showed the potential of XlnR engineering to develop robust T. amestolkiae strains for the valorisation of hemicellulosic residues.
Hide AbstractStructural and functional insights into extreme thermal stability and activity of two GH 12 domains of a multidomain glycosidase from a hyperthermophilic euryarchaeon.
Zayulina, K. S., Frolov, E. N., Stracke, C., Klyukina, A. A., Khusnutdinova, A. N., Stogios, P., Skarina, T., Yakunin, A., Golyshin, P. N., Siebers, B., Shugaeva, T. E. & Kublanov, I. V. (2025). The FEBS Journal, 70095.
Bacteria and fungi are well known for efficient degradation of plant polysaccharides thanks to various enzymes involved in plant cell wall decomposition. However, little is known about the role of archaea in this process or the repertoire and features of their polysaccharide-degrading enzymes. In our previous work, we discovered an archaeal multidomain glycosidase (MDG) composed of three catalytic domains (GH5 and two GH12) and two cellulose-binding modules (CBM2). The recombinant MDG and individual GH5 catalytic domain were active against cellulose and a number of other polysaccharides at a wide range of temperatures, with optimum temperatures (Topt) of 60°C and 80°C, respectively. The present study was focused on the characterization of two GH12 domains of the MDG. Purified recombinant TMDG_GH12-1 and TMDG_GH12-2 proteins were active as individual enzymes but exhibited distinct catalytic properties. Both enzymes were thermostable and active at extremely high temperatures: TMDG_GH12-1 was active at 40-130°C (Topt 100°C), and its half-life (t½) at 100°C was 42 h, which makes it one of the most thermostable glycosidases known so far, whereas TMDG_GH12-2 was active at 50-100°C (Topt 90°C) with t½ at 100°C being 30 min. Phylogenetic and structural analysis of both TMDG_GH12 proteins together with molecular docking and site-directed mutagenesis suggested that the presence of two disulfide bridges and the W → Q mutation in the active site contribute to the exceptional thermostability of TMDG_GH12-1. Further structural and mutational studies of the TMDG_GH12-1 domain will help to gain a better understanding of the molecular mechanisms of its extraordinary thermostability and substrate specificity.
Hide AbstractDetoxification of coumarins by rumen anaerobic fungi: insights into microbial degradation pathways and agricultural applications.
Li, Y., Gao, J., Cao, Y., Cheng, X., Sun, Z., Zhang, J., Zhu, W., Gierus, M. & Cheng, Y. (2025). Journal of Animal Science and Biotechnology, 16(1), 59.
Background: Coumarins are toxic phytochemicals found in a variety of plants and are known to limit microbial degradation and interfere with nutrient cycling. While the degradation of coumarins by fungi has been studied in an environmental context, little is known about their degradation in the gastrointestinal system of herbivores after ingestion. Results: In this study, we investigated in vitro fermentation by microbial enrichment, transcriptome sequencing, and high-resolution mass spectrometry to evaluate the ability of rumen anaerobic fungi to degrade coumarins. The results showed that despite the low abundance of anaerobic fungi in the rumen microbiota, they were able to effectively degrade coumarins. Specifically, Pecoramyces ruminantium F1 could tolerate coumarin concentrations up to 3 mmol/L and degrade it efficiently via metabolic pathways involving alpha/beta hydrolases and NAD(P)H oxidoreductases within the late growth phase. The fungus metabolized coumarin to less toxic compounds, including o-coumaric acid and melilotic acid, highlighting the detoxification potential of anaerobic fungi. Conclusions: This study is the first to demonstrate the ability of rumen anaerobic fungi to degrade coumarin, providing new insights into the use of anaerobic fungi in sustainable agricultural practices and environmental detoxification strategies.
Hide AbstractExpression and characterization of cold-adapted xylanase Xyl-L in Pichia pastoris for xylooligosaccharide (XOS) preparation.
Rodríguez, S., González, C., Reyes-Godoy, J. P., Gasser, B., Andrews, B. & Asenjo, J. A. (2025). Microbial Cell Factories, 24(1), 82.
Background: Xylan, the second most abundant polysaccharide in plant biomass, requires endoxylanases for its hydrolysis into xylooligosaccharides (XOS). Xylanases have been widely used in industries such as animal feed, bakery, juice production, and paper pulp. Recently, XOS have gained attention for their health benefits, including improved digestion, reduced cholesterol, and antioxidant effects. The cold-adapted GH10 xylanase of Antarctic origin Xyl-L was previously expressed in Escherichia coli, showing promising low-temperature activity. However, Pichia pastoris is currently a preferred host for industrial xylanase production due to its ability to express complex proteins and secrete them into the culture medium. This study explored the expression of Xyl-L in P. pastoris and evaluated its potential for XOS production using common flours as substrates, aiming for applications in the food and nutraceutical industry. Results: Comparison between AOX1 () and GAP () promoters for recombinant Xyl-L production in P. pastoris showed that the promoter resulted in higher activity per wet-cell weight. Co-transforming -Xyl strains with plasmids encoding genes aiding in protein folding (HAC1 or PDI1) did not enhance Xyl-L catalytic activity compared to the parental strain. Thus, -Xyl was cultivated in 3 L bioreactors in fed-batch cultures; it is presumed that the enzyme is produced with glycosylations within its structure, given its migration within the SDS-PAGE gels. The produced Xyl-L was purified from the culture supernatant, resulting in peak xylanase activity after 90 h, with specific activity of 5.10 ± 0.21 U/mg, at pH 7.5 and C, using beechwood xylan. It also showed a Km of 3.5 mg/mL and a kcat of 9.16 . Xyl-L maintained over 80% of relative activity between pH 5.68.6 and C, and was activated by and , but inhibited by . Xyl-L was tested using several flours (whole wheat, rye, oatmeal and all-purpose) as substrates, where XOS with a polymerization degree (DP) of 2 were obtained from each substrate, whole wheat flour generated XOS with DP 3, and XOS with DP 2, 3 and 4 were produced when beechwood xylan was used as substrate. Conclusions: The xylanase Xyl-L was successfully expressed in P. pastoris and proved to be able to degrade various flour substrates, producing XOS with DP ranging from 2 to 4, indicating its potential applications in the nutraceutical and food industries. Further studies must be performed to optimize its production in bioreactors.
Hide AbstractSynergy of GH67 and GH115 α-1, 2-glucuronidases with Penicillium subrubescens endoxylanases to stimulate xylooligosaccharide production.
Li, X., Li, L., Manassero, A., Müller, A., Reddy, S. K., Kabel, M. A., de Vries, R. P. & Sun, P. (2025). Enzyme and Microbial Technology, 187, 110629.
A primary substitution of the plant cell wall hemicellulosic polysaccharide xylan is (4-O-methyl-)d-glucuronic acid, which hinders the endoxylanases (XLNs) degradation of xylan for the production of valuable xylooligosaccharides (XOS). In this context, α-1,2-glucuronidase (AGU) plays a critical role in hydrolyzing the α-(1→2)-glycosidic linkages between 4-O-methyl-d-glucuronic acid and xylosyl residues in xylan, thereby enhancing XOS production by XLNs. However, AGUs have been relatively poorly studied, and insufficient and incomplete data on their biochemical properties, substrate specificity, and product profiling has limited their application. Here, we cloned, heterologously produced, purified and functionally characterized an AGU from Aspergillus niger (AnAguA) and another AGU from Penicillium subrubescens (PsAguB), belonging to Glycoside Hydrolase family 67 (GH67) and 115 (GH115), respectively, in the Carbohydrate-Active enZyme database. Results showed that neither AGU released 4-O-methyl-d-glucuronic acid from polymeric beech wood glucuronoxylan (BeWX). However, we found that from BeWX pre-digested with GH10 or GH11 XLNs from P. subrubescens (PsXlnA and PsXlnF, respectively), AnAguA released 4-O-methyl-d-glucuronic acid only from the non-reducing end of glucuronoxylan oligosaccharide, whereas PsAguB released 4-O-methyl-d-glucuronic acid from glucuronoxylan oligosaccharides regardless of the xylosyl substitution position. Furthermore, we demonstrated that enhancement of XOS release by adding AGUs to various combinations of GH10 (PsXlnA–C) and GH11 (PsXlnD–F, PsXlnH–I) XLNs from P. subrubescens varied based on the AGU-XLN combination. The combination of AnAguA with PsXlnA was the most effective, achieving at least a 3-fold increase in the release of XOS with a degree of polymerization of 5-7 compared to using PsXlnA alone.
Hide AbstractReassigning the role of a mesophilic xylan hydrolysing family GH43 β-xylosidase from Bacteroides ovatus, BoExXyl43A as exo-β-1, 4-xylosidase.
Gavande, P. V., Ji, S., Cardoso, V., Fontes, C. M. & Goyal, A. (2024). Current Research in Biotechnology, 7, 100191.
The recombinant 40 kDa BoExXyl43A glycoside hydrolase family 43 (GH43) from bacterium Bacteroides ovatus exhibited highest specific activity (U/mg) against corn cob xylan (136.8), followed by Beechwood xylan (81.1), Carbosynth xylan (69.3), 4-O-D-methylglucuronoxylan (61.4) and Birchwood xylan (59.9). BoExXyl43A demonstrated optimal performance at 37 °C and pH 7.6 with Vmax and Km of 141.8 U/mg and 4.0 mg/mL as well as 64.1 U/mg and 6.0 mg/mL against corn cob and Birchwood xylan, respectively. The activity of BoExXyl43A increased by 48 % by addition of 10 mM Ca2+ ions, while 1 mM EDTA or 1 mM EGTA decreased its activity by 100 % or 42.5 %, respectively, highlighting its calcium-ion dependence. Thin-layer chromatography (TLC) analysis of BoExXyl43A hydrolysates of Birchwood and Beechwood xylan as well as that of various xylooligosaccharides (DP2-DP9) from corn cob xylan showed the release of D-xylose, identifying it as an exo-β-1,4-xylosidase/exo-β-1,4-xylanase (EC 3.2.1.-/3.2.1.37). Moreover, the time-dependent TLC analysis of xylobiose hydrolysis showed release of D-xylose units, confirming its β-xylosidase activity. BoExXyl43A also exhibited exo-1,4-β-xylosidase activity on Larchwood and Carbosynth xylans. Notably, it released D-xylose from α-L-Araf2-xylotriose demonstrating its activity against decorated xylooligosaccharides. BoExXyl43A's exo-1,4-β-xylosidase and residual β-xylosidase activity on xylan and xylobiose, respectively, could potentially enhance xylan saccharification efficiency in bioethanol-based refineries. The molecular modeling showed that BoExXyl43A has 5-bladed β-propeller structure with a very shallow active-site having −1, +1 and + 2 subsites, which could accommodate three D-xylose units of longer xylan like xylododecaose thus supporting its exoxylosidase activity.
Hide AbstractImprovement of rice noodle quality by saturated-steam heat moisture treatment.
Yan, X., Luo, S., Ye, J. & Liu, C. (2025). Carbohydrate Polymers, 123303.
Heat moisture treatment (HMT) of starch granules is a successful technique for enhancing rice noodle quality; however, conventional HMT is time-consuming. In this study, efficient saturated-steam HMT (SS-HMT) was employed for gel modification to enhance rice noodle quality. This treatment was performed under saturated steam (produced under atmospheric pressure in a water bath at 100°C) for brief durations (5, 10, 15, and 20 min), and the underlying mechanism was investigated by examining the variation in starch multiscale structures. SS-HMT disrupted the short double helices and single helices and promoted the formation of longer double helices through rearrangement, increasing the network tie-point size and starch thermal stability. High thermal stability reduced starch leaching and minimized damage to the gas cell walls during cooking, resulting in thicker gas cell walls that enhanced the samples' mechanical strength. SS-HMT markedly improved rice noodle quality. Compared with the control group, rice noodles treated with SS-HMT for 10 min exhibited a 56.05% reduction in cooking loss, a 100% decrease in breakage rate, a 48.46 % increase in hardness, and a 24.68% decrease in adhesiveness. This study provides a straightforward and efficient strategy for improving rice noodle quality.
Hide AbstractDPANN symbiont of Haloferax volcanii accelerates xylan degradation by the non-host haloarchaeon Halorhabdus sp.
Reva, O. N., La Cono, V., Marturano, L., Crisafi, F., Smedile, F., Mudaliyar, M., Ghosal, D., Selivanova, E. A., Ignatenko, M. E., Ferrer, M., Fernandez-Lopez, L., Krupovic, M. & Yakimov, M. M. (2025). iScience, 28(2).
This study examines a natural consortium of halophilic archaea, comprising xylan-degrading Halorhabdus sp. SVX81, consortium cohabitant Haloferax volcanii SVX82 (formerly H. lucentense SVX82), and its DPANN ectosymbiont Ca. Nanohalococcus occultus SVXNc. Transcriptomics and targeted metabolomics demonstrated that the tripartite consortium outperformed individual and the Halorhabdus sp. SVX81 with H. volcanii SVX82 bipartite cultures in xylan degradation, exhibiting a division of labor: the DPANN symbiont processed glycolysis products, while other members performed xylan depolymerization and biosynthesis of essential compounds. Electron microscopy and cryo-electron tomography revealed the formation of heterocellular biofilms interlinked by DPANN cells. The findings demonstrated that DPANN symbionts can interact directly with other members of microbial communities, which are not their primary hosts, influencing their gene expression. However, DPANN proliferation requires their primary host presence. The study highlights the collective contribution of consortium members to xylan degradation and their potential for biotechnological applications in the management of hypersaline environments.
Hide Abstract