100 assays (manual) / 1000 assays (microplate) / 1300 assays (auto-analyser)
| Content: | 100 assays (manual) / 1000 assays (microplate) / 1300 assays (auto-analyser) |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
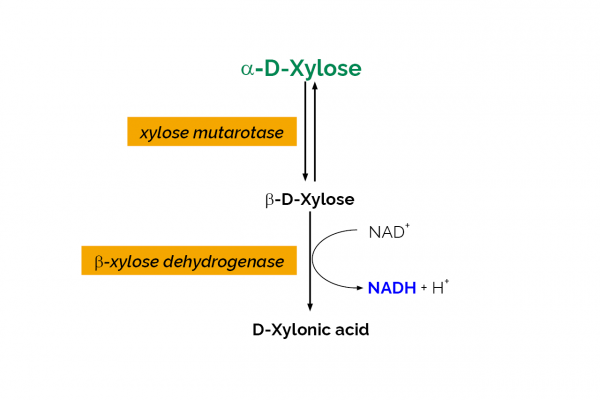
| Analyte: | D-Xylose |
| Assay Format: | Spectrophotometer, Microplate, Auto-analyser |
| Detection Method: | Absorbance |
| Wavelength (nm): | 340 |
| Signal Response: | Increase |
| Linear Range: | 2 to 100 μg of D-xylose per assay |
| Limit of Detection: | 0.7 mg/L |
| Reaction Time (min): | ~ 6 min |
| Application examples: | Analysis of D-xylose in fermentation broths and hydrolysates of plant material and polysaccharides. |
| Method recognition: | Novel method |
The D-Xylose test kit is a novel method for the specific, convenient and rapid measurement and analysis of D-xylose in plant extracts, culture media/supernatants and other materials.
Note for Content: The number of manual tests per kit can be doubled if all volumes are halved. This can be readily accommodated using the MegaQuantTM Wave Spectrophotometer (D-MQWAVE).
View our full range of monosaccharide assay kits.

- Very cost effective
- All reagents stable for > 2 years after preparation
- Only enzymatic kit available
- Rapid reaction (~ 6 min)
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
- Suitable for manual, microplate and auto-analyser formats
Measurement of available carbohydrates in cereal and cereal products, dairy products, vegetables, fruit and related food products and animal feeds: First Action 2020.07.
McCleary, B. V. & McLoughlin, C. (2021). Journal of AOAC International, qsab019.
Background: The level of available carbohydrates in our diet is directly linked to two major diseases; obesity and Type II diabetes. Despite this, to date there is no method available to allow direct and accurate measurement of available carbohydrates in human and animal foods. Objective: The aim of this research was to develop a method that would allow simple and accurate measurement of available carbohydrates, defined as non-resistant starch, maltodextrins, maltose, isomaltose, sucrose, lactose, glucose, fructose and galactose. Method: Non-resistant (digestible) starch is hydrolysed to glucose and maltose by pancreatic α-amylase and amyloglucosidase at pH 6.0 with shaking or stirring at 37°C for 4 h. Sucrose, lactose, maltose and isomaltose are completely hydrolyzed by specific enzymes to their constituent monosaccharides, which are then measured using pure enzymes in a single reaction cuvette. Results: A method has been developed that allows the accurate measurement of available carbohydrates in all cereal, vegetable, fruit, food, and feed products, including dairy products. Conclusions: A single-laboratory validation was performed on a wide range of food and feed products. The inter-day repeatability (%RSDr) was <3.58% (w/w) across a range of samples containing 44.1 to 88.9% available carbohydrates. The LOD and LOQ obtained were 0.054% (w/w) and 0.179% (w/w), respectively. The method is all inclusive, specific, robust and simple to use. Highlights: A unique method has been developed for the direct measurement of available carbohydrates, entailing separate measurement of glucose, fructose and galactose; information of value in determining the glycemic index of foods.
Hide AbstractImprovement of β‐Xylosidase and Endoxylanase Activities in Talaromyces amestolkiae by Genetic Manipulation of the Transcriptional Activator XlnR.
Pozo‐Rodríguez, A., Peñalva, M. Á., Barriuso, J., Espeso, E. A. & Martínez, M. J. (2025). Microbial Biotechnology, 18(5), e70166.
The ascomycete Talaromyces amestolkiae is a promising source of glycosyl hydrolases for hemicellulose degradation, as it contains a considerably higher number of genes encoding these enzymes than other fungi exploited for plant biomass valorisation. The development of genetic engineering tools could further improve its biotechnological potential. We report here a transformation system for T. amestolkiae based on pyrimidine auxotrophy complementation, which was used to successfully introduce both integrative and autonomously replicating plasmids. Then, we applied this tool to force the expression of the transcriptional activator XlnR, generating an engineered strain with enhanced β‐xylosidase (1.4‐fold) and endoxylanase (2.0‐fold) activities compared to the wild‐type cultured on xylan. Markedly larger improvements were obtained after introducing Ala788Val or Val785Phe substitutions in XlnR, achieving 3.3‐fold and 3.9‐fold increases in β‐xylosidase and endoxylanase activities, respectively, in the case of XlnRV785F. This recombinant strain also displays a partial deregulation of the hemicellulolytic system when cultivated on glucose and glycerol (a low‐cost and renewable substrate), yielding notably higher production of β‐xylosidases (16.9‐fold and 13.8‐fold) and endoxylanases (31.9‐fold and 22.7‐fold) than the wild‐type. Increased efficiencies of XlnRV785F enzymatic crudes in xylan saccharifications showed the potential of XlnR engineering to develop robust T. amestolkiae strains for the valorisation of hemicellulosic residues.
Hide AbstractGut microbiota modulation and inflammation mitigation in a murine model through a hull-less and purple grain barley genotype.
Cortijo-Alfonso, M. E., Laghouaouta, H., Pena, R. N., Martínez, M., Yuste, S., Rubió-Piqué, L. & Piñol-Felis, C. (2025). Food & Function, 16(6), 2389-2400.
Barley, increasingly recognized for its health benefits, contains bioactive compounds like beta-glucans and (poly)phenols. Newly developed purple barley varieties, enriched with anthocyanins, offer potential gut health benefits. This study examined the effects of a hull-less, purple-grain barley genotype, consumed as whole-grain or isolated fractions (bran and endosperm), on gut microbiota and inflammation in a murine model. Fifty male and female BALB/cB&J mice were assigned to five diets over six weeks: standard diet (SD), rice diet (RD), whole-grain barley (WGB), anthocyanin-rich barley bran (BB), and beta-glucan-rich endosperm (PG). The BB diet triggered anti-inflammatory signals as it reduced IFN-γ and IL-4 in females, lowered TNF-α in both sexes, and decreased C-Reactive Protein (CRP) in males compared to SD. The PG diet improved gut barrier integrity by lowering LPS-binding protein levels. Barley-based diets enhanced gut microbiota diversity, particularly, by increasing beneficial bacteria like Lactobacillus, Lachnospiraceae UCG-001, and Akkermansia. Notably, BB and PG elicited stronger effects than WGB, suggesting that grain fractionation modifies the food matrix, potentially enhancing the bioaccessibility and bioavailability of key bioactive compounds. These results underscore the benefits of purple barley-derived fractions in promoting gut health and reducing inflammation, supporting their potential role to protect against inflammation-related conditions.
Hide AbstractThe role of MYB46 in modulating polysaccharide acetylation by mediating the transcriptional regulation of cell wall-related genes in Arabidopsis.
Rastogi, L., Deshpande, S. & Pawar, P. A. M. (2025). BioRxiv, 2025-01.
Understanding the mechanism behind the transcriptional regulation of polysaccharide O-acetylation remains a key challenge that might be regulated through transcription factors. Our earlier work revealed the upregulation of AtGELP7 in MYB46 overexpression lines, prompting us to investigate how MYB46 transcriptionally controls AtGELP7 including other cell wall acetylation pathway genes in Arabidopsis. In MYB46 overexpression lines, we observed alteration in acetylation levels on xylan, xyloglucan and pectin in different tissue types, which suggests complex and tight regulation of acetylation homeostasis in the cell wall. Further, our transcriptomic data revealed the simultaneous upregulation of both AtGELP7 (acetyl xylan esterase) and xylan-specific TBLs (xylan O-acetyltransferases) indicating sophisticated regulation of cell wall acetylation homeostasis. Using transactivation studies in Nicotiana and pAtGELP7::GUS stable lines, we found that MYB46 enhances AtGELP7 expression probably through downstream regulators which could be either MYB103 or other MYBs. In addition, extensive cell wall analysis of MYB46 overexpression lines showed differential sugar distribution, preferably to major cell wall components such as cellulose, xyloglucan, and xylan across different tissue types of different developmental stages. Moreover, the integration of RNA-sequencing and ChIP-sequencing data uncovered previously unknown, novel probable direct gene targets of MYB46 that may be involved in polysaccharide acetylation and cell wall remodeling.
Hide AbstractMetabolic bottlenecks of Pseudomonas taiwanensis VLB120 during growth on D-xylose via the Weimberg pathway.
Nerke, P., Korb, J., Haala, F., Hubmann, G. & Lütz, S. (2024). Metabolic Engineering Communications, 18, e00241.
The microbial production of value-added chemicals from renewable feedstocks is an important step towards a sustainable, bio-based economy. Therefore, microbes need to efficiently utilize lignocellulosic biomass and its dominant constituents, such as D-xylose. Pseudomonas taiwanensis VLB120 assimilates D-xylose via the five-step Weimberg pathway. However, the knowledge about the metabolic constraints of the Weimberg pathway, i.e., its regulation, dynamics, and metabolite fluxes, is limited, which hampers the optimization and implementation of this pathway for bioprocesses. We characterized the Weimberg pathway activity of P. taiwanensis VLB120 in terms of biomass growth and the dynamics of pathway intermediates. In batch cultivations, we found excessive accumulation of the intermediates D-xylonolactone and D-xylonate, indicating bottlenecks in D-xylonolactone hydrolysis and D-xylonate uptake. Moreover, the intermediate accumulation was highly dependent on the concentration of D-xylose and the extracellular pH. To encounter the apparent bottlenecks, we identified and overexpressed two genes coding for putative endogenous xylonolactonases PVLB_05820 and PVLB_12345. Compared to the control strain, the overexpression of PVLB_12345 resulted in an increased growth rate and biomass generation of up to 30% and 100%, respectively. Next, D-xylonate accumulation was decreased by overexpressing two newly identified D-xylonate transporter genes, PVLB_18545 and gntP (PVLB_13665). Finally, we combined xylonolactonase overexpression with enhanced uptake of D-xylonate by knocking out the gntP repressor gene gntR (PVLB_13655) and increased the growth rate and biomass yield by 50 % and 24% in stirred-tank bioreactors, respectively. Our study contributes to the fundamental knowledge of the Weimberg pathway in pseudomonads and demonstrates how to encounter the metabolic bottlenecks of the Weimberg pathway to advance strain developments and cell factory design for bioprocesses on renewable feedstocks.
Hide AbstractCombination of alkaline biodiesel-derived crude glycerol pretreated corn stover with dilute acid pretreated water hyacinth for highly-efficient single cell oil production by oleaginous yeast Cutaneotrichosporon oleaginosum.
Liu, Y., Zhou, W., Zhao, M., Ma, Q., Zhang, J., Zhou, W. & Gong, Z. (2024). Bioresource Technology, 395, 130366.
Single cell oil (SCO) prepared from biodiesel-derived crude glycerol (BCG) and lignocellulosic biomass (LCB) via oleaginous yeasts is an intriguing alternative precursor of biodiesel. Here, a novel strategy combining alkaline BCG pretreated corn stover and dilute acid pretreated water hyacinth for SCO overproduction was developed. The mixed pretreatment liquors (MPLs) were naturally neutralized and adjusted to a proper carbon-to-nitrogen ratio beneficial for SCO overproduction by Cutaneotrichosporon oleaginosum. The toxicity of inhibitors was relieved by dilution detoxification. The enzymatic hydrolysate of solid fractions was suitable for SCO production either separately or simultaneously with MPLs. Fed-batch fermentation of the MPLs resulted in high cell mass, SCO content, and SCO titer of 80.7 g/L, 75.7%, and 61.1 g/L, respectively. The fatty acid profiles of SCOs implied high-quality biodiesel characteristics. This study offers a novel BCG&LCB-to-SCO route integrating BCG-based pretreatment and BCG/LCB hydrolysates co-utilization, which provides a cost-effective technical route for micro-biodiesel production.
Hide AbstractIsolation and characterization of microalgae strains able to grow on complex biomass hydrolysate for industrial application.
Badary, A., Hidasi, N., Ferrari, S. & Mayfield, S. P. (2024). Algal Research, 78, 103381.
Producing algae biomass economically remains one of the chief bottlenecks for commercializing algae products. The aim of this work is to identify new strains of algae that can grow on cost effective media derived from cellulosic waste streams, characterize the potential of these strains to produce compounds of high industrial value, and identify those strains capable of facile genetic transformation. Here we report, out of 45 strains initially isolated, three were selected based on their ability to efficiently grow on organic waste material (corn stover hydrolysate) as a carbon source; Chlorococcum sp., Desmodesmus sp., and Chlamydomonas debaryana. Untargeted metabolomics was performed on each strain, identifying several metabolites of high relative abundances that are of commercial interest, such as lactic acid, butane-2,3-diol, amino acids, tartaric acid, triacylglycerols, and lipid species containing different, mono- and polyunsaturated fatty acids, depending on the strain and growth condition. The strains also produced carbohydrates of industrial relevance. Chlorococcum sp. was found to be genetically transformable using standard simple transformation protocols. These results suggest that with further development, these strains could open the door to economic production of high value commercial compounds utilizing waste streams from cellulosic biomass.
Hide AbstractSimultaneous production of dry solid biomass and liquid extract from Sorghum bicolor using liquefied ammonia.
Sakuragi, K., Tokunaga, T. & Otaka, M. (2024). Bioresource Technology Reports, 25, 101757.
Energy-consuming processes should be avoided while separating biomass components. This study investigated the dewatering and extraction of components from Sorghum bicolor silage using liquefied NH3 (NH3(l)). Using a plug-flow-type reactor, NH3(l) was passed through the specimen at 20°C and 0.85 MPa. When the NH3/wet sample weight was 4.7, a 31.0 wt% dry ammonia-treated sample (AT) was obtained as an extract, achieving a 96.5 wt% dewatering ratio. Glucose, xylose, and an acid-insoluble fraction were retained in the AT, whereas lactic and acetic acids were separated as an extract. Crystalline cellulose was transformed, and the increase in glucose and xylose yields from the enzymatic hydrolysis of the AT was similar levels to those from sulfuric acid hydrolysis. Therefore, NH3(l) treatment can dewater S. bicolor silage without severe pretreatment. In addition, it can be used in biofuel and chemical production using enzymatically degradable ATs from which organic acids are separated as extracts.
Hide AbstractHighly-efficient lipid production from hydrolysate of Radix paeoniae alba residue by oleaginous yeast Cutaneotrichosporon oleaginosum.
Xu, C., Wang, Y., Zhang, C., Liu, J., Fu, H., Zhou, W. & Gong, Z. (2024). Bioresource Technology, 391, 129990.
Valorization of herbal extraction residues (HERs) into value-added products is pivotal for the sustainability of Chinese medicine industry. Here, seven different enzymatic hydrolysates of dilute acid pretreated HERs were evaluated for lipid production by Cutaneotrichosporon oleaginosum. Among them, the highest sugar yield via hydrolysis and the maximum lipid production were obtained from Radix paeoniae alba residue (RPAR). More interestingly, high proportion of sugar polymers was disintegrated into fermentable sugars during the pretreatment step, allowing a cheap non-enzymatic route for producing sugars from RPAR. A repeated dilute acid pretreatment gained a high sugar concentration of 241.6 g/L through reusing the pretreatment liquor (PL) for four times. Biomass, lipid concentration, and lipid content achieved 49.5 g/L, 35.7 g/L and 72.2 %, respectively, using fed-batch culture of PL. The biodiesel parameters indicated lipids produced from HERs were suitable for biodiesel production. This study offers a cost-effective way to upgrade the HERs waste into micro-biodiesel.
Hide AbstractXylobiose treatment triggers a defense-related response and alters cell wall composition.
Dewangan, B. P., Gupta, A., Sah, R. K., Das, S., Kumar, S., Bhattacharjee, S. & Pawar, P. A. M. (2023). Plant Molecular Biology, 113(6), 383-400.
Plant cell wall-derived oligosaccharides, i.e., damage-associated molecular patterns (DAMPs), could be generated after pathogen attack or during normal plant development, perceived by cell wall receptors, and can alter immunity and cell wall composition. Therefore, we hypothesised that xylo-oligosaccharides (XOS) could act as an elicitor and trigger immune responses. To test this, we treated Arabidopsis with xylobiose (XB) and investigated different parameters. XB-treatment significantly triggered the generation of reactive oxygen species (ROS), activated MAPK protein phosphorylation, and induced callose deposition. The combination of XB (DAMP) and flg22 a microbe-associated molecular pattern (MAMP) further enhanced ROS response and gene expression of PTI marker genes. RNA sequencing analysis revealed that more genes were differentially regulated after 30 min compared to 24 h XB-treated leaves, which correlated with ROS response. Increased xylosidase activity and soluble xylose level after 30 min and 3 h of XB-treatment were observed which might have weakened the DAMP response. However, an increase in total cell wall sugar and a decrease in uronic acid level was observed at both 30 min and 24 h. Additionally, arabinose, rhamnose, and xylose levels were increased in 30 min, and glucose was increased in 24 h compared to mock-treated leaves. The level of jasmonic acid, abscisic acid, auxin, and cytokinin were also affected after XB treatment. Overall, our data revealed that the shortest XOS can act as a DAMP, which triggers the PTI response and alters cell wall composition and hormone level.
Hide AbstractRecovery of Nanocellulose from Agri-Food Residues through Chemical and Physical Processes.
Pirozzi, A., Pappalardo, G. & Donsì, F. (2023). Chemical Engineering Transactions, 102, 175-180.
This work proposes a biorefinery approach for the exploitation of agri-food by-products, such as tomato pomace (TP), through the combination of mild chemical hydrolysis and high-pressure homogenization (HPH) in water not only to promote the recovery of cellulose but also its defibrillation to obtain nanocellulose. In particular, the cellulose pulp was isolated from TP using different combinations of chemical and physical processes, by applying HPH treatment (i) directly on the raw material, (ii) after the acid hydrolysis, and (iii) after alkaline hydrolysis. Moreover, the isolated cellulose was deconstructed to obtain cellulose nanoparticles, also through the application of the HPH treatment, enhancing the polymer properties. The structural and physical features of cellulose nanoparticles from TP were analyzed through Fourier-transform infrared spectroscopy (FT-IR) analysis, ?-potential measurement, and morphological analysis with SEM. The results clearly showed that the HPH treatment (80 MPa, 20 passes) at different stages of the process caused only a slight increase in the yield of cellulose recovery, but significantly contributed to obtaining defibrillated cellulose particles, characterized by smaller irregular domains containing elongated needle-like fibers.
Hide AbstractEngineering transcriptional regulation of pentose metabolism in Rhodosporidium toruloides for improved conversion of xylose to bioproducts.
Coradetti, S. T., Adamczyk, P. A., Liu, D., Gao, Y., Otoupal, P. B., Geiselman, G. M., Webb-Robertson, B. J. M., Burnet, M. C., Kim, Y. M., Burnum-Johnson, K. E., Magnuson, J. & Gladden, J. M. (2023). Microbial Cell Factories, 22(1), 144.
Efficient conversion of pentose sugars remains a significant barrier to the replacement of petroleum-derived chemicals with plant biomass-derived bioproducts. While the oleaginous yeast Rhodosporidium toruloides (also known as Rhodotorula toruloides) has a relatively robust native metabolism of pentose sugars compared to other wild yeasts, faster assimilation of those sugars will be required for industrial utilization of pentoses. To increase the rate of pentose assimilation in R. toruloides, we leveraged previously reported high-throughput fitness data to identify potential regulators of pentose catabolism. Two genes were selected for further investigation, a putative transcription factor (RTO4_12978, Pnt1) and a homolog of a glucose transceptor involved in carbon catabolite repression (RTO4_11990). Overexpression of Pnt1 increased the specific growth rate approximately twofold early in cultures on xylose and increased the maximum specific growth by 18% while decreasing accumulation of arabitol and xylitol in fast-growing cultures. Improved growth dynamics on xylose translated to a 120% increase in the overall rate of xylose conversion to fatty alcohols in batch culture. Proteomic analysis confirmed that Pnt1 is a major regulator of pentose catabolism in R. toruloides. Deletion of RTO4_11990 increased the growth rate on xylose, but did not relieve carbon catabolite repression in the presence of glucose. Carbon catabolite repression signaling networks remain poorly characterized in R. toruloides and likely comprise a different set of proteins than those mainly characterized in ascomycete fungi.
Hide AbstractThe effect of different wheat varieties and exogenous xylanase on bird performance and utilization of energy and nutrients.
Whiting, I. M., Pirgozliev, V. & Bedford, M. R. (2023). Poultry Science, 102817.
The aims of the present study were to first, determine the xylan fractions of 10 different wheat cultivar samples and their response to treatment by the same commercial xylanase enzyme preparation. Second, use information obtained to select 5 of the wheats for use within a feeding experiment to determine whether the rate of xylan release can be used to predict the feeding value of the wheats when diets have been supplemented with xylanase. Treatment of 10 different wheat varieties by the same enzyme resulted in varying levels of hydrolysis. Soluble xylan content ranged from 7.85 to 14.40 and 3.20 to 5.13 (mg/g) when treated with and without xylanase, respectively. Oligosaccharide content ranged from 0.34 to 1.58 and 0.05 to 0.54 (mg/g) when treated with and without xylanase, respectively. Five of the 10 wheats were then selected based on the determined xylan fractions to use within a feeding experiment. A total of 360 male Ross 308 broilers were randomly allocated to 60 raised floor pens. A soybean meal (SBM) balancer feed was formulated to contain 12.07 MJ/kg apparent metabolizable energy (AME) and 392.9 g/kg crude protein (CP). Five diets were prepared by mixing 630 g/kg of each of the 5 experimental wheats with 370 g/kg of the balancer. Each diet was split into 2, one of which was supplemented with 100 g/MT of Econase XT (223,000 BXU/g), resulting in a total of 10 diets. The birds were fed the diets from 0 to 28 d of age. Wheat cultivar had an effect (P = 0.044) on feed intake (FI), while the addition of xylanase increased (P < 0.05) weight gain (WG) and improved feed conversion ratio (FCR). Various interactions were observed (P < 0.05) between wheat cultivars and xylanase for AME and nutrient utilization. This study suggests that wheats treated with the same xylanase, differ in their susceptibility to release soluble xylan and oligosaccharides, which may partially explain the varying performance and nutrient digestibility responses noted in the literature.
Hide AbstractGreen synthesis of nickel ferrite nanoparticles for efficient enhancement of lignocellulosic hydrolysate-based biohydrogen production.
Zhang, Q., Cao, J., Zhao, P., Zhang, Y., Li, Y., Xu, S., Ye, C. & Qian, C. (2023). Biochemical Engineering Journal, 194, 108885.
To improve the lignocellulosic hydrolysate-based production of biohydrogen, bimetallic nickel ferrite nanoparticles (NPs) with small particle size, cubic shape, high stability, and biocompatibility are synthesized using an Eichhornia crassipes extract at a low annealing temperature of 400 °C and added to the lignocellulosic hydrolysate fermentation by Klebsiella sp. WL1316. The optimal addition of 30 mg/L gsNiFe2O4 NPs accounts for the highest cumulative hydrogen production of 5544.86 ± 37.03 mL/L and improvement of 112.32% ± 1.86% at 24 h, while also resulting in the highest improvement of hydrogenase and formate-hydrogen lyase activities up to 102.11% ± 13.73% and 62.99% ± 4.66% compared to the Control treatment, respectively. Moreover, the conversion efficiencies of glucose, xylose, and substrate are enhanced upon addition of gsNiFe2O4 NPs, reaching values higher than 96% in the presence of 30 mg/L gsNiFe2O4 NPs. At the same time, the hydrogen yield converted from the substrate (Y(H2/S)) and biomass converted from the substrate (Y(B/S)) are also improved. In addition, the alteration of soluble metabolic products, especially significant changes in formic acid and ethanol concentrations compared to the control, increases the flux in the formate-hydrogen lytic pathway for hydrogen evolution, thereby promoting the substrate conversion level to hydrogen gas.
Hide AbstractMonthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei, Formerly Palmaria palmata in Japan).
Yamamoto, R., Mune Mune, M. A., Miyabe, Y., Kishimura, H. & Kumagai, Y. (2023). Phycology, 3(1), 127-137.
Mycosporine-like amino acids (MAAs) are natural ultraviolet-absorbing compounds found in microalgae and macroalgae. MAA content changes seasonally and in response to environmental factors. We previously investigated MAAs from the red alga dulse (Devaleraea inkyuleei, formerly Palmaria palmata in Japan) in Usujiri, Hokkaido, Japan, from 2019 to 2020. At that time, some factors affecting MAA content were still unclear. In this study, we investigated MAA variation during the period from January to June 2021, and evaluated new methods of MAA extraction from dulse. We recorded a maximum MAA extraction yield (7.03 µmol/g dry weight) on 25 March 2021. Over the course of our three years of investigations from 2019 to 2021, we found that dulse was most suitable for MAA preparation from the middle of February to late April. In the later work reported in this paper, we improved our extraction method by using a lower-risk organic solvent (ethanol) rather than methanol. In addition, we evaluated MAA extraction using different levels of ethanol concentration (25, 50, and 99%) and different extraction times (2, 6, and 24 h). We found that extraction with 25% ethanol for 24 h increased MAA content by a factor of 3.2, compared with our previous extraction method. In summary, we determined the most suitable sampling period for Usujiri dulse, to extract the highest content of MAAs. We also improved the effectiveness of the extraction process.
Hide Abstract