| Content: | 100 assays per kit |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | Fructan |
| Assay Format: | Spectrophotometer |
| Detection Method: | Absorbance |
| Wavelength (nm): | 410 |
| Signal Response: | Increase |
| Linear Range: | 2.3 to 55 µg of D-fructose or D-glucose per assay |
| Limit of Detection: | 0.16 g/100 g |
| Total Assay Time: | ~ 90 min |
| Application examples: | Flours, infant formula, animal feed, pet foods, plant materials (e.g. onion), food products and other materials |
| Method recognition: | AACC Method 32-32.01, AOAC Method 999.03, AOAC Method 2016.14, AOAC Method 2018.07 and CODEX Method Type III |
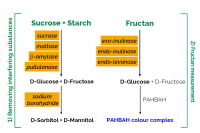
The Fructan Assay Kit is suitable for the specific measurement of fructan in plant extracts, animal feed and food products containing starch, sucrose and other sugars. It is used in three validated methods for the determination of fructan: AOAC method 999.03 (foods), AOAC method 2018.07 (Animal Feed) and AOAC method 2016.14 (infant formula and adult nutritionals).
New, improved procedure.
In the most recent development, a recombinant endo-levanase has been incorporated into the fructanase mixture, extending the use of the method to the measurement of levan-type fructans as are present in grasses such as timothy, cocksfoot, ryegrass and red fescue.
The method described in this booklet employs ultra-pure, recombinant enzymes and specifically measures fructans including inulin-type fructans from chicory, dahlia, jerusalem artichoke; highly branched fructans from onion and wheat stems and leaves; and levan-type fructans from pasture grasses such as timothy grass. The enzymes employed are completely devoid of contaminating enzymes active on β-glucan or gluco-oligosaccharides.
Browse our full range of polysaccharide assay kits.

- Very cost effective
- All kit reagents stable for > 2 years after preparation
- Unaffected by high sucrose / reducing sugar concentrations
- Fructan kits are only available from Megazyme
- Simple format
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
Determination of Fructan (Inulin, FOS, Levan, and Branched Fructan) in Animal Food (Animal Feed, Pet Food, and Ingredients): Single-Laboratory Validation, First Action 2018.07.
McCleary, B. V., Charmier, L. M. J., McKie, V. A., Ciara McLoughlin, C. & Rogowski, A. (2019). Journal of AOAC International, 102(3), 2019 883.
Traditional enzyme-based methods for measurement of fructan were designed to measure just inulin and branched-type (agave) fructans. The enzymes employed, namely exo-inulinase and endo-inulinase, give incompletely hydrolysis of levan. Levan hydrolysis requires a third enzyme, endo-levanase. This paper describes a method and commercial test kit (Megazyme Fructan Assay Kit) for the determination of all types of fructan (inulin, levan, and branched) in a variety of animal feeds and pet foods. The method has been validated in a single laboratory for analysis of pure inulin, agave fructan, levan, and a range of fructan containing samples. Quantification is based on complete hydrolysis of fructan to fructose and glucose by a mixture of exo-inulinase, endo-inulinase, and endo-levanase, followed by measurement of these sugars using the PAHBAH reducing sugar method which gives the same color response with fructose and glucose. Before hydrolysis of fructan, interfering sucrose and starch in the sample are specifically hydrolyzed and removed by borohydride reduction. The single-laboratory validation (SLV) outlined in this document was performed on commercially available inulin (Raftiline) and agave fructan (Frutafit©), levan purified from Timothy grass, two grass samples, a sample of legume hay, two animal feeds and two barley flours, one of which (Barley MAX©) was genetically enriched in fructan through plant breeding. Parameters examined during the validation included working range, target selectivity, recovery, LOD, LOQ, trueness (bias), precision (repeatability and intermediate precision), robustness, and stability. The method is robust, quick, and simple.
Hide AbstractMcCleary, B. V., Charnock, S. J., Rossiter, P. C., O’Shea, M. F., Power, A. M. & Lloyd, R. M. (2006). Journal of the Science of Food and Agriculture, 86(11), 1648-1661.
Procedures for the measurement of starch, starch damage (gelatinised starch), resistant starch and the amylose/amylopectin content of starch, β-glucan, fructan, glucomannan and galactosyl-sucrose oligosaccharides (raffinose, stachyose and verbascose) in plant material, animal feeds and foods are described. Most of these methods have been successfully subjected to interlaboratory evaluation. All methods are based on the use of enzymes either purified by conventional chromatography or produced using molecular biology techniques. Such methods allow specific, accurate and reliable quantification of a particular component. Problems in calculating the actual weight of galactosyl-sucrose oligosaccharides in test samples are discussed in detail.
Hide AbstractMeasurement of total fructan in foods by enzymatic/spectrophotometric method: Collaborative study.
McCleary, B. V., Murphy, A. & Mugford, D. C. (2000). Journal of AOAC International, 83(2), 356-364.
An AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measuring oligofructans and fructan polysaccharide (inulins) in mixed materials and food products. The sample is extracted with hot water, and an aliquot is treated with a mixture of sucrase (a specific sucrose-degrading enzyme), α-amylase, pullulanase, and maltase to hydrolyze sucrose to glucose and fructose, and starch to glucose. These reducing sugars are then reduced to sugar alcohols by treatment with alkaline borohydride solution. The solution is neutralized, and excess borohydride is removed with dilute acetic acid. The fructan is hydrolyzed to fructose and glucose using a mixture of purified exo- and endo-inulinanases (fructanase mixture). The reducing sugars produced (fructose and glucose) are measured with a spectrophotometer after reaction with para-hydroxybenzoic acid hydrazide. The samples analyzed included pure fructan, chocolate, low-fat spread, milk powder, vitamin tablets, onion powder, Jerusalem artichoke flour, wheat stalks, and a sucrose/cellulose control flour. Repeatability relative standard deviations ranged from 2.3 to 7.3%; reproducibility relative standard deviations ranged from 5.0 to 10.8%.
Hide AbstractMcCleary, B. V., Gibson, T. S. & Mugford, D. C. (1997). Journal of AOAC International, 80, 571-579.
An American Association of Cereal Chemists/AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measurement of total starch in a range of cereal grains and products. The flour sample is incubated at 95 degrees C with thermostable alpha-amylase to catalyze the hydrolysis of starch to maltodextrins, the pH of the slurry is adjusted, and the slurry is treated with a highly purified amyloglucosidase to quantitatively hydrolyze the dextrins to glucose. Glucose is measured with glucose oxidase-peroxidase reagent. Thirty-two collaborators were sent 16 homogeneous test samples as 8 blind duplicates. These samples included chicken feed pellets, white bread, green peas, high-amylose maize starch, white wheat flour, wheat starch, oat bran, and spaghetti. All samples were analyzed by the standard procedure as detailed above; 4 samples (high-amylose maize starch and wheat starch) were also analyzed by a method that requires the samples to be cooked first in dimethyl sulfoxide (DMSO). Relative standard deviations for repeatability (RSD(r)) ranged from 2.1 to 3.9%, and relative standard deviations for reproducibility (RSD(R)) ranged from 2.9 to 5.7%. The RSD(R) value for high amylose maize starch analyzed by the standard (non-DMSO) procedure was 5.7%; the value was reduced to 2.9% when the DMSO procedure was used, and the determined starch values increased from 86.9 to 97.2%.
Hide AbstractUnraveling the functional potential of microbial resources and pulse-based matrices for sourdough breadmaking.
Viretto, C., Tlais, A. Z. A., Arora, K., Ameur, H., Tuccillo, F., Polo, A., Ardèvol., V. N., Verté., F., Katina., K., Di Cagno, R. & Gobbetti, M. (2025). Future Foods, 11, 100643.
This study aimed to optimize sourdough preparation from pulse-based flours (faba bean flour, faba bean protein concentrate, and yellow pea flour) using several binary consortia of lactic acid bacteria and yeasts for making new bread formulations. A total of 288 type-II sourdoughs with varying flour substrates and fermentation times were designed. Screening based on optimal sourdough criteria identified seventeen best-performing type-II sourdoughs. The transition to type-IV sourdoughs markedly enhanced their maturity and functionality. Six most promising pulse-based type-IV sourdoughs were selected as tailored inocula for sourdough breadmaking, leading to significant biochemical differences compared to wholewheat bread made with baker's yeast (control). Pulse-based sourdough breads showed higher protein content and superior amino acid profiles. The lowest levels of antinutritional factors were found when Leuconostoc mesenteroides and Saccharomyces cerevisiae were co-cultured in yellow pea. Despite lower in vitro protein digestibility in sourdough breads, the synergistic interaction of Levilactobacillus namurensis and Torulaspora delbrueckii improved protein quality regardless of flour compositions. Additionally, pulse-based sourdoughs enriched the breads with phenolics, like catechin, rutin, epicatechin, sinapic acid, and quercetin, offering substantial functional benefits. Despite the adverse textural characteristics, most sourdough breads exhibited more complex volatile organic compound profiles compared to that of the control.
Hide AbstractPrebiotic potential of oligosaccharides extracted from improved Ugandan varieties of millet, sesame, soybean, and sorghum: enhancing probiotic growth and enteric pathogen inhibition.
Alowo, D., Olum, S., Mukisa, I. M. & Ongeng, D. (2025). BMC microbiology, 25(1), 1-17.
Functional gastrointestinal disorders like diarrhea continue to affect children under five years in low-income countries. Incorporating health-enhancing bioactive compounds such as prebiotics in diet offers a promising solution. This study investigated prebiotic potential of oligosaccharides extracted from improved varieties of millet (Seremi 2, Naromil 2), sesame (Sesim 2, Sesim 3), soybean (Maksoy 3N, Maksoy 6N), and sorghum (Narosorg 2, Narosorg 4), commonly consumed in Uganda. These were compared to their respective indigenous variety. This study employed standardized methods for optical density measurement, culture preparation, and oligosaccharide extraction to evaluate prebiotic properties. We investigated whether plant-based oligosaccharides could enhance the effectiveness of probiotics, specifically Lactiplantibacillus plantarum (ATCC 14917) and Lacticaseibacillus rhamnosus (ATCC 7469), in antagonizing common enteric pathogens (Salmonella enterica subsp. enterica (ATCC 13076) and Shigella flexneri (ATCC 12022)). Approximately 4-8 log CFU/ml of each probiotic was incubated in 2% w/v oligosaccharide extracts at 37°C to evaluate the influence of the extracts on their growth, short-chain fatty acid (SCFA) production and antagonistic activity. Maximum cell density, which exceeded the minimum recommended probiotic cell density (6 log CFU/ml), was achieved during 24-h incubation period. The probiotics exhibited optimal growth in extracts of Sesim 2, Maksoy 3N, Narosorg 2 and indigenous millet variety resulting in a 68-84% increase in cell densities. The concentration of SCFA concentration was significantly higher (p < 0.05) in soybean-based oligosaccharides. Both probiotics antagonized growth of Salmonella and Shigella by more than 40% when cultured on Sesim 2, Maksoy 3N, Narosorg 2 and indigenous millet variety, while maintaining the probiotic cell densities above the minimum recommended level. These varieties show great potential as functional ingredients for developing synbiotic-rich foods to promote gut and public health. However, to evaluate the oligosaccharides prebiotic efficacy, in vitro fermentation using fecal microbiota and in vivo studies are necessary to determine gut microbiota changes and interactions.
Hide AbstractEffect of thermal and non-thermal processing on fermentable oligo-di-monosaccharides and polyols (FODMAPs) content in millet, sorghum, soybean and sesame varieties.
Alowo, D., Olum, S., Mukisa, I. M. & Ongeng, D. (2025). Frontiers in Nutrition, 12, 1520510.
This study investigated the effect of processing (roasting and malting) and crop variety on fermentable oligo-di-monosaccharides and polyols (FODMAPs) profile of millet, sorghum, soybean, and sesame varieties commonly consumed in Uganda. Two elite varieties and one indigenous variety for each crop were analyzed. Monosaccharide and polyols content was determined by HPLC-UV method, while disaccharides and oligosaccharide were determined using Megazyme kits. The elite varieties of soybean (Maksoy 3 N), Millet (Seremi 2) and sorghum (Narosorg 2) exhibited significantly (p < 0.05) lower oligosaccharide content compared to indigenous varieties with percentage differences ranging from 10.2 to 73.9%. Additionally, Maksoy 3 N and Narosorg 2 also exhibited significantly lower (p < 0.05) excess fructose content compared to the indigenous variety. Malting was more effective than roasting (p < 0.05) in reducing FODMAP categories and total FODMAP content. Malting effectively reduced excess fructose in all grain types to the recommended levels of <0.15 g/100 g compared to roasting. Moreover, malting reduced total oligosaccharides and total FODMAPs in soybean and sesame by more than 50%. However, this reduction did not achieve the recommended threshold of 0.3 g/100 g for total oligosaccharides and 0.5 g/100 g, for total FODMAPs which are a criterion to categorize low FODMAP diets. Malting conditions should be optimized to enhance its effectiveness in producing low FODMAP foods. This study highlights the importance of selecting appropriate grain variety and processing techniques that modify FODMAP content in foods that can be used for dietary therapy of gastro-intestinal disorders among vulnerable population.
Hide AbstractBiotechnological Tools for the Production of Low-FODMAP Wholegrain Wheat and Rye Cookies and Crackers.
Torbica, A. M., Filipčev, B., Vujasinović, V., Miljić, U., Radivojević, G., Miljić, M. & Radosavljević, M. (2025). Foods, 14(4), 582.
Fermentable oligosaccharides, di- and monosaccharides, and polyols defined as FODMAPs readily trigger the symptoms of irritable bowel syndrome (IBS), which affects up to 23% of the population, through several mechanisms. A low-FODMAP diet is a short-term solution due to significant nutrient deficiencies, especially in dietary fibre (DF). IBS patients must avoid cereals, especially wholegrain cereals such as wheat and rye, which are an important natural source of DF and therefore FODMAPs (part of soluble DF). This study is the first of its kind to employ biotechnological tools for the creation of wholegrain low-FODMAP cookies and crackers based on wholegrain wheat and rye flours with high FODMAP contents. Endogenous enzymes activated via prolonged dough resting and exogenously activated enzymes originating from chicory extract, wheat malt, and baker’s yeast were employed. The prolonged dough resting time and the addition of wheat malt reduced the FODMAP content in the wholegrain wheat and rye cookies by 46% and 99.5%, respectively. The best result was achieved in the wholegrain wheat crackers, with a FODMAP content reduction of 59.3% based on the combination of a prolonged dough resting time and the addition of wheat malt and baker’s yeast. In the wholegrain rye crackers, a prolonged resting time alone was sufficient to achieve an 83.6% reduction in the total oligosaccharide content.
Hide AbstractAustralian wheat cultivar gluten/gliadin sensitivity illustrates potential for celiac-safe wheat.
Yousif, A. M., Florides, C. G., Zhou, M., Riaz, Q., Békés, F. & Eri, R. (2025). Journal of Cereal Science, 122, 104105.
Wheat and wheat products are very popular in the general population. However, incidents of non-celiac gluten sensitivity (NCGS), gluten ataxia, neuro-psychiatric disorders wheat allergy and coeliac disease are observed to be on the increase. 11 varieties of wheat were assessed for gliadin and glutenin content and protein composition via SE-HPLC, RP-HPLC, Maldi-TOF, fructans and in vitro assays for assessing the efficacy of available cultivar fractions. Out of the 47-69 gliadin proteins, all 11 cultivars displayed 5 gliadin proteins and 43 polypeptides in one cultivar. Glutenin content was found to be 42-46% for all cultivars except one. Cytotoxicity assays in CaCo2 cells demonstrated correlations between gliadin composition and cellular toxicity, with average levels of cytotoxicity for gliadin and glutenin at 10.7% and 16.3% respectively. α-gliadin showing the strongest link to increased cytotoxicity (Cultivars with lower gliadin content exhibited reduced cytotoxicity, highlighting their potential in breeding programs). The findings underscore the feasibility of selecting wheat varieties with significantly lower toxic components while maintaining functional properties. Although modern breeding practices have not eliminated CD epitopes, they present opportunities to develop cultivars with minimized antigenicity. Future studies should expand cytotoxicity analyses using multiple cell lines or human organoids to deepen understanding and enhance breeding strategies. These insights can guide the production of safer wheat varieties for sensitive populations and inform sustainable agricultural practices.
Hide AbstractChallenges to Increasing Dietary Fiber in White Flour and Bread.
Shewry, P. R., Prins, A., Kosik, O. & Lovegrove, A. (2024). Journal of Agricultural and Food Chemistry, 72(24).
Increasing the intake of dietary fiber from staple foods is a key strategy to improve the health of consumers. White bread is an attractive vehicle to deliver increased fiber as it is widely consumed and available to all socio-economic groups. However, fiber only accounts for about 4% of the dry weight of white flour and bread compared to 10-15% in whole grain bread and flour. We therefore discuss the challenges and barriers to developing and exploiting new types of wheat with high fiber content in white flour. These include defining and quantifying individual fiber components and understanding how they are affected by genetic and environmental factors. Rapid high throughput assays suitable for determining fiber content during plant breeding and in grain-utilizing industries are urgently required, while the impact of fiber amount and composition on flour processing quality needs to be understood. Overcoming these challenges should have significant effects on human health.
Hide AbstractInhibitory effects of burdock root tea on plasma ammonia level in mice fed with high-sucrose and low-fibre diet.
Sato, M., Kuda, T., Yamamoto, M., Nakamura, A., Takahashi, H., Inoue, J. & Takayanagi, S. (2024). Food Bioscience, 59, 104186.
Roasted burdock root tea (BT), rich in inulin and chlorogenic acid, is currently being established as a tea beverage in Japan and a wider area. This study aimed to clarify the effects of BT on the gut environment and host health. BT was prepared using 5% (w/v) dried burdock root, and its effect on ammonia production was investigated using human faecal culture and Institute of Cancer Research mice fed with a high-sucrose and low-dietary fibre diet for 14 days. In human faecal cultures established using a medium prepared with BT, the pH significantly decreased from 6.5 to 4.6 ± 0.1. In vitro, BT significantly suppressed the production of ammonia and indole (p < 0.05). In vivo, BT increased the caecal acetate level from 34 ± 3 μmol/g to 49 ± 4 μmol/g and n-butyrate level from 5.1 ± 0.7 μmol/g to 14 ± 1 μmol/g and reduced the caecal pH from 7.0 ± 0.1 to 6.6 ± 0.1. Despite no significant changes in caecal ammonia levels, plasma ammonia levels in BT-treated mice decreased from 0.74 ± 0.07 to 0.50 ± 0.07 μmol/mL. Moreover, 16S rDNA (V4) amplicon sequencing of faeces revealed that BT increased the short-chain fatty acid-producing gut commensals Muribaculaceae and Clostridia UCG-014. These results suggest that BT has desirable functional properties correlated with short-chain fatty acid production and pH-lowering effects that inhibit ammonia production and absorption in the gut.
Hide AbstractIn vitro and ex vivo metabolism of chemically diverse fructans by bovine rumen Bifidobacterium and Lactobacillus species.
King, M. L., Xing, X., Reintjes, G., Klassen, L., Low, K. E., Alexander, T. W., Waldner, M., Patel, T. R. & Wade Abbott, D. (2024). Animal Microbiome, 6(1), 50.
Background: Inulin and inulin-derived fructooligosaccharides (FOS) are well-known prebiotics for use in companion animals and livestock. The mechanisms by which FOS contribute to health has not been fully established. Further, the fine chemistry of fructan structures from diverse sources, such as graminan-type fructans found in cereal crops, has not been fully elucidated. New methods to study fructan structure and microbial responses to these complex carbohydrates will be key for evaluating the prebiotic potency of cereal fructans found in cattle feeds. As the rumen microbiome composition is closely associated with their metabolic traits, such as feed utilization and waste production, prebiotics and probiotics represent promising additives to shift the microbial community toward a more productive state. Results: Within this study, inulin, levan, and graminan-type fructans from winter wheat, spring wheat, and barley were used to assess the capacity of rumen-derived Bifidobacterium boum, Bifidobacterium merycicum, and Lactobacillus vitulinus to metabolize diverse fructans. Graminan-type fructans were purified and structurally characterized from the stems and kernels of each plant. All three bacterial species grew on FOS, inulin, and cereal crop fructans in pure cultures. L. vitulinus was the only species that could metabolize levan, albeit its growth was delayed. Fluorescently labelled polysaccharides (FLAPS) were used to demonstrate interactions with Gram-positive bacteria and confirm fructan metabolism at the single-cell level; these results were in agreement with the individual growth profiles of each species. The prebiotic potential of inulin was further investigated within naïve rumen microbial communities, where increased relative abundance of Bifidobacterium and Lactobacillus species occurred in a dose-dependent and temporal-related manner. This was supported by in situ analysis of rumen microbiota from cattle fed inulin. FLAPS probe derived from inulin and fluorescent in situ hybridization using taxon-specific probes confirmed that inulin interacts with Bifidobacteria and Lactobacilli at the single-cell level. Conclusion: This research revealed that rumen-derived Bifidobacteria and Lactobacilli vary in their metabolism of structurally diverse fructans, and that inulin has limited prebiotic potential in the rumen. This knowledge establishes new methods for evaluating the prebiotic potential of fructans from diverse plant sources as prebiotic candidates for use in ruminants and other animals.
Hide AbstractCharacterization and optimization of continuous ohmic thermal sterilization based on the development of a predictive computational toolbox.
Rivera, J., Gratz, M., Jaeger, H. & Schottroff, F. (2024). Innovative Food Science & Emerging Technologies, 96, 103792.
Continuous thermal processing (CTP) is a common method for sterilizing food. However, it can result in an uneven temperature distribution, which can lead to a varying degree of processing intensity. Ohmic heating (OH) can be advantageous in this regard, as it enables volumetric heating for more homogenous treatments. However, evaluating the processing intensity distribution inside the equipment for OH is challenging due to the complex interaction between electrical, mechanical and thermal phenomena. Furthermore, the comparison of OH and conventional heating treatments often lack a profound basis of comparable treatment intensity considerations. To gain a deeper mechanistic understanding of the technology, a numerical computational fluid dynamics model for the OH sterilization of a clear carrot juice from the heating region to the cooling process was developed. The model was validated with thermal and electrical measurements and showed an error rate below 2.5% in its prediction capacities. Moreover, the model was implanted for the validation of the products sterilization and compared to a conventional validation approach, reviling a 33.3% underestimation of the thermal load by conventional manners, which can lead to faulty sterilization of the food product. Additionally, the model was expanded to also be able to predict the microbial inactivation ratio of the system with an average error of 1.10±0.74%. In addition, results indicate that the numerical calculation of the F0 values and their validation with the microbial inactivation ratio have a notable potential for localization and evaluation of hotspots in OH simulations. Therefore, it can be seen as a promising step for establishing a foundation for computer-assisted optimization of CTP and targeted processing.
Hide AbstractDetermination of prebiotic activity and probiotic encapsulation ability of inulin type fructans obtained from Inula helenium roots.
Meral, H. D., Özcan, F. Ş., Özcan, N., Bozkurt, F. & Sağdiç, O. (2024). Journal of Food Science, 89(9), 5335-5349.
Inulin, a prebiotic utilized in the food and pharmaceutical industries, promotes the growth of beneficial bacteria in the colon, thereby enhancing human health. Although inulin is commercially produced from chicory and artichoke, Inula helenium roots offer a high potential for inulin production. The aim of this study is to investigate the prebiotic activity of inulin (inulin-P) from I. helenium roots on Lactobacillus rhamnosus, as well as its ability to produce synbiotic microcapsules and the effects on probiotic viability during freeze-drying, in vitro gastrointestinal (GI) digestion, and storage. First, the effect of inulin-P on L. rhamnosus viability and short-chain fatty acid (SCFA) production was compared to other commonly utilized prebiotics. The findings revealed that inulin-P remarkably promoted the growth and SCFA yield of L. rhamnosus for 48 h of fermentation and 28 days of storage. Then, L. rhamnosus was encapsulated with inulin-P and commercial inulin to compare its survival throughout storage and the GI tract. Inulin-P microcapsules outperformed in terms of viability during storage (7.98 log CFU/g after 30 days at 4°C). Furthermore, inulin-P microcapsules were heat-resistant and protected L. rhamnosus from GI conditions, resulting in a high survival rate (89.52%) following large intestine simulation, which is ideal for increasing customer benefits. Additionally, inulin-P microcapsules exhibited similar physical characteristics to commercial inulin. Consequently, this study revealed that inulin-P, which is easy to produce, low-cost, and has industrial application potential, could be used as a good carrier for the synbiotic encapsulation of L. rhamnosus.
Hide AbstractIdentification of inulin-responsive bacteria in the gut microbiota via multi-modal activity-based sorting.
Riva, A., Rasoulimehrabani, H., Cruz-Rubio, J. M., Schnorr, S. L., von Baeckmann, C., Inan, D., et al. (2023). Nature Communications, 14(1), 8210.
Prebiotics are defined as non-digestible dietary components that promote the growth of beneficial gut microorganisms. In many cases, however, this capability is not systematically evaluated. Here, we develop a methodology for determining prebiotic-responsive bacteria using the popular dietary supplement inulin. We first identify microbes with a capacity to bind inulin using mesoporous silica nanoparticles functionalized with inulin. 16S rRNA gene amplicon sequencing of sorted cells revealed that the ability to bind inulin was widespread in the microbiota. We further evaluate which taxa are metabolically stimulated by inulin and find that diverse taxa from the phyla Firmicutes and Actinobacteria respond to inulin, and several isolates of these taxa can degrade inulin. Incubation with another prebiotic, xylooligosaccharides (XOS), in contrast, shows a more robust bifidogenic effect. Interestingly, the Coriobacteriia Eggerthella lenta and Gordonibacter urolithinfaciens are indirectly stimulated by the inulin degradation process, expanding our knowledge of inulin-responsive bacteria.
Hide Abstract