| Content: |
0.5 grams - 10 mL or 2 grams - 40 mL or 5 grams - 100 mL or 2.5 grams - 100 mL (ANKOM) |
| Shipping Temperature: | Ambient |
| Storage Temperature: | 2-8oC |
| Formulation: | In 50% (v/v) glycerol |
| Physical Form: | Solution |
| Stability: | > 1 year under recommended storage conditions |
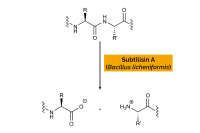
| Enzyme Activity: | Protease |
| EC Number: | 3.4.21.62 |
| CAS Number: | 9014-01-1 |
| Synonyms: | subtilisin; subtilisin A |
| Source: | Bacillus licheniformis |
| Molecular Weight: | 30,250 |
| Expression: | Purified from Bacillus licheniformis |
| Specificity: | Hydrolysis of proteins with broad specificity for peptide bonds, and a preference for a large uncharged residue in P1. Hydrolyses peptide amides. |
| Specific Activity: | ~ 6 U/mg of protein (40oC, pH 8.0 on casein) |
| Unit Definition: | One Unit will hydrolyse casein to produce colour equivalent to one μmole (181 µg) of tyrosine per minute at pH 7.0 at 40oC (colour by Folin-Ciocalteu reagent). |
| Temperature Optima: | 60oC |
| pH Optima: | 7 |
| Application examples: | This enzyme is recommended for use in the Megazyme Total Dietary Fiber test method and AOAC INTERNATIONAL Total Dietary Fiber analytical procedures. |
The E-BSPRT-10ML pack size has been discontinued (read more).
High purity Protease (Subtilisin A from Bacillus licheniformis) (liquid) for use in research, biochemical enzyme assays and analytical testing applications.
For use in Megazyme Total Dietary Fiber test method.
E-BSPRT-A-100ML specifically to be used with ANKOMTDF Dietary Fiber Analyzer.
Show all analytical enzymes products.
Data booklets for each pack size are located in the Documents tab.
Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products.
McCleary, B. V., McLoughlin, C., Charmier, L. M. J. & McGeough, P. (2019). Cereal Chemistry, 97(1), 114-137.
Background and objectives: The importance of selectively measuring available and unavailable carbohydrates in the human diet has been recognized for over 100 years. The levels of available carbohydrates in diets can be directly linked to major diseases of the Western world, namely Type II diabetes and obesity. Methodology for measurement of total carbohydrates by difference was introduced in the 1880s, and this forms the basis of carbohydrate determination in the United States. In the United Kingdom, a method to directly measure available carbohydrates was introduced in the 1920s to assist diabetic patients with food selection. The aim of the current work was to develop simple, specific, and reliable methods for available carbohydrates and digestible starch (and resistant starch). The major component of available carbohydrates in most foods is digestible starch. Findings: Simple methods for the measurement of rapidly digested starch, slowly digested starch, total digestible starch, resistant starch, and available carbohydrates have been developed, and the digestibility of phosphate cross‐linked starch has been studied in detail. The resistant starch procedure developed is an update of current procedures and incorporates incubation conditions with pancreatic α‐amylase (PAA) and amyloglucosidase (AMG) that parallel those used AOAC Method 2017.16 for total dietary fiber. Available carbohydrates are measured as glucose, fructose, and galactose, following complete and selective hydrolysis of digestible starch, maltodextrins, maltose, sucrose, and lactose to glucose, fructose, and galactose. Sucrose is hydrolyzed with a specific sucrase enzyme that has no action on fructo‐oligosaccharides (FOS). Conclusions: The currently described “available carbohydrates” method together with the total dietary fiber method (AOAC Method 2017.16) allows the measurement of all carbohydrates in food products, including digestible starch. Significance and novelty: This paper describes a simple and specific method for measurement of available carbohydrates in cereal, food, and feed products. This is the first method that provides the correct measurement of digestible starch and sucrose in the presence of FOS. Such methodology is essential for accurate labeling of food products, allowing consumers to make informed decisions in food selection.
Hide AbstractModification to AOAC Official Methods 2009.01 and 2011.25 to allow for minor overestimation of low molecular weight soluble dietary fiber in samples containing starch.
McCleary, B. V. (2014). Journal of AOAC International, 97(3), 896-901.
AOAC Official Methods 2009.01 and 2011.25 have been modified to allow removal of resistant maltodextrins produced on hydrolysis of various starches by the combination of pancreatic α-amylase and amyloglucosidase (AMG) used in these assay procedures. The major resistant maltodextrin, 63,65-di-α-D-glucosyl maltopentaose, is highly resistant to hydrolysis by microbial α-glucosidases, isoamylase, pullulanase, pancreatic, bacterial and fungal α-amylase and AMG. However, this oligosaccharide is hydrolyzed by the mucosal α-glucosidase complex of the pig small intestine (which is similar to the human small intestine), and thus must be removed in the analytical procedure. Hydrolysis of these oligosaccharides has been by incubation with a high concentration of a purified AMG at 60°C. This incubation results in no hydrolysis or loss of other resistant oligosaccharides such as FOS, GOS, XOS, resistant maltodextrins (e.g., Fibersol 2) or polydextrose. The effect of this additional incubation with AMG on the measured level of low molecular weight soluble dietary fiber (SDFS) and of total dietary fiber in a broad range of samples is reported. Results from this study demonstrate that the proposed modification can be used with confidence in the measurement of dietary fiber.
Hide AbstractMeasurement of total dietary fiber using AOAC method 2009.01 (AACC International approved method 32-45.01): Evaluation and updates.
McCleary, B. V., Sloane, N., Draga, A. & Lazewska, I. (2013). Cereal Chemistry, 90(4), 396-414.
The Codex Committee on Methods of Analysis and Sampling recently recommended 14 methods for measurement of dietary fiber, eight of these being type I methods. Of these type I methods, AACC International Approved Method 32-45.01 (AOAC method 2009.01) is the only procedure that measures all of the dietary fiber components as defined by Codex Alimentarius. Other methods such as the Prosky method (AACCI Approved Method 32-05.01) give similar analytical data for the high-molecular-weight dietary fiber contents of food and vegetable products low in resistant starch. In the current work, AACCI Approved Method 32-45.01 has been modified to allow accurate measurement of samples high in particular fructooligosaccharides: for example, fructotriose, which, in the HPLC system used, chromatographs at the same point as disaccharides, meaning that it is currently not included in the measurement. Incubation of the resistant oligosaccharides fraction with sucrase/β-galactosidase removes disaccharides that interfere with the quantitation of this fraction. The dietary fiber value for resistant starch type 4 (RS4), varies significantly with different analytical methods, with much lower values being obtained with AACCI Approved Method 32-45.01 than with 32-05.01. This difference results from the greater susceptibility of RS4 to hydrolysis by pancreatic α-amylase than by bacterial α-amylase, and also a greater susceptibility to hydrolysis at lower temperatures. On hydrolysis of samples high in starch in the assay format of AACCI Approved Method 32-45.01 (AOAC method 2009.01), resistant maltodextrins are produced. The major component is a heptasaccharide that is highly resistant to hydrolysis by most of the starch-degrading enzymes studied. However, it is hydrolyzed by the maltase/amyloglucosidase/isomaltase enzyme complex present in the brush border lining of the small intestine. As a consequence, AOAC methods 2009.01 and 2011.25 (AACCI Approved Methods 32-45.01 and 32-50.01, respectively) must be updated to include an additional incubation with amyloglucosidase to remove these oligosaccharides.
Hide AbstractDetermination of insoluble, soluble, and total dietary fiber (codex definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative Study.
McCleary, B. V., DeVries, J. W., Rader, J. I., Cohen, G., Prosky, P., Mugford, D. C., Champ, M. & Okuma, K. (2012). Journal of AOAC International, 95(3), 824-844.
A method for the determination of insoluble (IDF), soluble (SDF), and total dietary fiber (TDF), as defined by the CODEX Alimentarius, was validated in foods. Based upon the principles of AOAC Official MethodsSM 985.29, 991.43, 2001.03, and 2002.02, the method quantitates water-insoluble and water-soluble dietary fiber. This method extends the capabilities of the previously adopted AOAC Official Method 2009.01, Total Dietary Fiber in Foods, Enzymatic-Gravimetric-Liquid Chromatographic Method, applicable to plant material, foods, and food ingredients consistent with CODEX Definition 2009, including naturally occurring, isolated, modified, and synthetic polymers meeting that definition. The method was evaluated through an AOAC/AACC collaborative study. Twenty-two laboratories participated, with 19 laboratories returning valid assay data for 16 test portions (eight blind duplicates) consisting of samples with a range of traditional dietary fiber, resistant starch, and nondigestible oligosaccharides. The dietary fiber content of the eight test pairs ranged from 10.45 to 29.90%. Digestion of samples under the conditions of AOAC 2002.02 followed by the isolation, fractionation, and gravimetric procedures of AOAC 985.29 (and its extensions 991.42 and 993.19) and 991.43 results in quantitation of IDF and soluble dietary fiber that precipitates (SDFP). The filtrate from the quantitation of water-alcohol-insoluble dietary fiber is concentrated, deionized, concentrated again, and analyzed by LC to determine the SDF that remains soluble (SDFS), i.e., all dietary fiber polymers of degree of polymerization = 3 and higher, consisting primarily, but not exclusively, of oligosaccharides. SDF is calculated as the sum of SDFP and SDFS. TDF is calculated as the sum of IDF and SDF. The within-laboratory variability, repeatability SD (Sr), for IDF ranged from 0.13 to 0.71, and the between-laboratory variability, reproducibility SD (sR), for IDF ranged from 0.42 to 2.24. The within-laboratory variability sr for SDF ranged from 0.28 to 1.03, and the between-laboratory variability sR for SDF ranged from 0.85 to 1.66. The within-laboratory variability sr for TDF ranged from 0.47 to 1.41, and the between-laboratory variability sR for TDF ranged from 0.95 to 3.14. This is comparable to other official and approved dietary fiber methods, and the method is recommended for adoption as Official First Action.
Hide AbstractDetermination of total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study.
McCleary, B. V., DeVries, J. W., Rader, J. I., Cohen, G., Prosky, L., Mugford, D. C., Champ, M. & Okuma, K. (2010). Journal of AOAC International, 93(1), 221-233.
A method for the determination of total dietary fiber (TDF), as defined by the CODEX Alimentarius, was validated in foods. Based upon the principles of AOAC Official MethodsSM 985.29, 991.43, 2001.03, and 2002.02, the method quantitates high- and low-molecular-weight dietary fiber (HMWDF and LMWDF, respectively). In 2007, McCleary described a method of extended enzymatic digestion at 37°C to simulate human intestinal digestion followed by gravimetric isolation and quantitation of HMWDF and the use of LC to quantitate low-molecular-weight soluble dietary fiber (LMWSDF). The method thus quantitates the complete range of dietary fiber components from resistant starch (by utilizing the digestion conditions of AOAC Method 2002.02) to digestion resistant oligosaccharides (by incorporating the deionization and LC procedures of AOAC Method 2001.03). The method was evaluated through an AOAC collaborative study. Eighteen laboratories participated with 16 laboratories returning valid assay data for 16 test portions (eight blind duplicates) consisting of samples with a range of traditional dietary fiber, resistant starch, and nondigestible oligosaccharides. The dietary fiber content of the eight test pairs ranged from 11.57 to 47.83. Digestion of samples under the conditions of AOAC Method 2002.02 followed by the isolation and gravimetric procedures of AOAC Methods 985.29 and 991.43 results in quantitation of HMWDF. The filtrate from the quantitation of HMWDF is concentrated, deionized, concentrated again, and analyzed by LC to determine the LMWSDF, i.e., all nondigestible oligosaccharides of degree of polymerization 3. TDF is calculated as the sum of HMWDF and LMWSDF. Repeatability standard deviations (Sr) ranged from 0.41 to 1.43, and reproducibility standard deviations (SR) ranged from 1.18 to 5.44. These results are comparable to other official dietary fiber methods, and the method is recommended for adoption as Official First Action.
Hide AbstractMcCleary, B. V., Mills, C. & Draga, A. (2009). Quality Assurance and Safety of Crops & Foods, 1(4), 213–224.
An integrated total dietary fibre (TDF) method, consistent with the recently accepted CODEX definition of dietary fibre, has been developed. The CODEX Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU) has been deliberating for the past 8 years on a definition for dietary fibre that correctly reflects the current consensus thinking on what should be included in this definition. As this definition was evolving, it became evident to us that neither of the currently available methods for TDF (AOAC Official Methods 985.29 and 991.43), nor a combination of these and other methods, could meet these requirements. Consequently, we developed an integrated TDF procedure, based on the principals of AOAC Official Methods 2002.02, 991.43 and 2001.03, that is compliant with the new CODEX definition. This procedure quantitates high- and low-molecular weight dietary fibres as defined, giving an accurate estimate of resistant starch and non-digestible oligosaccharides also referred to as low-molecular weight soluble dietary fibre. In this paper, the method is discussed, modifications to the method to improve simplicity and reproducibility are described, and the results of the first rounds of interlaboratory evaluation are reported.
Hide AbstractAn integrated procedure for the measurement of total dietary fibre (including resistant starch), non-digestible oligosaccharides and available carbohydrates.
McCleary, B. V. (2007). Analytical and Bioanalytical Chemistry, 389(1), 291-308.
A method is described for the measurement of dietary fibre, including resistant starch (RS), non-digestible oligosaccharides (NDO) and available carbohydrates. Basically, the sample is incubated with pancreatic α-amylase and amyloglucosidase under conditions very similar to those described in AOAC Official Method 2002.02 (RS). Reaction is terminated and high molecular weight resistant polysaccharides are precipitated from solution with alcohol and recovered by filtration. Recovery of RS (for most RS sources) is in line with published data from ileostomy studies. The aqueous ethanol extract is concentrated, desalted and analysed for NDO by high-performance liquid chromatography by a method similar to that described by Okuma (AOAC Method 2001.03), except that for logistical reasons, D-sorbitol is used as the internal standard in place of glycerol. Available carbohydrates, defined as D-glucose, D-fructose, sucrose, the D-glucose component of lactose, maltodextrins and non-resistant starch, are measured as D-glucose plus D-fructose in the sample after hydrolysis of oligosaccharides with a mixture of sucrase/maltase plus β-galactosidase.
Hide AbstractMeasurement of novel dietary fibres.
McCleary, B. V. & Rossiter, P. (2004). Journal of AOAC International, 87(3), 707-717.
With the recognition that resistant starch (RS) and nondigestible oligosaccharides (NDO) act physiologically as dietary fiber (DF), a need has developed for specific and reliable assay procedures for these components. The ability of AOAC DF methods to accurately measure RS is dependent on the nature of the RS being analyzed. In general, NDO are not measured at all by AOAC DF Methods 985.29 or 991.43, the one exception being the high molecular weight fraction of fructo-oligosaccharides. Values obtained for RS, in general, are not in good agreement with values obtained by in vitro procedures that more closely imitate the in vivo situation in the human digestive tract. Consequently, specific methods for the accurate measurement of RS and NDO have been developed and validated through interlaboratory studies. In this paper, modifications to AOAC fructan Method 999.03 to allow accurate measurement of enzymically produced fructo-oligosaccharides are described. Suggested modifications to AOAC DF methods to ensure complete removal of fructan and RS, and to simplify pH adjustment before amyloglucosidase addition, are also described.
Hide AbstractMcCleary, B. V. (2003). Proceedings of the Nutrition Society, 62, 3-9.
The 'gold standard' method for the measurement of total dietary fibre is that of the Association of Official Analytical Chemists (2000; method 985.29). This procedure has been modified to allow measurement of soluble and insoluble dietary fibre, and buffers employed have been improved. However, the recognition of the fact that non-digestible oligosaccharides and resistant starch also behave physiologically as dietary fibre has necessitated a re-examination of the definition of dietary fibre, and in turn, a re-evaluation of the dietary fibre methods of the Association of Official Analytical Chemists. With this realisation, the American Association of Cereal Chemists appointed a scientific review committee and charged it with the task of reviewing and, if necessary, updating the definition of dietary fibre. It organised various workshops and accepted comments from interested parties worldwide through an interactive website. More recently, the (US) Food and Nutrition Board of the Institute of Health, National Academy of Sciences, under the oversight of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, assembled a panel to develop a proposed definition(s) of dietary fibre. Various elements of these definitions were in agreement, but not all. What was clear from both reviews is that there is an immediate need to re-evaluate the methods that are used for dietary fibre measurement and to make appropriate changes where required, and to find new methods to fill gaps. In this presentation, the 'state of the art' in measurement of total dietary fibre and dietary fibre components will be described and discussed, together with suggestions for future research.
Hide AbstractTwo issues in dietary fiber measurement.
McCleary, B. V. (2001). Cereal Foods World, 46, 164-165.
Enzyme activity and purity of these topics, the easiest to deal with is the importance of enzyme purity and activity. As a scientist actively involved in polysaccharide research over the past 25 years, I have come to appreciate the importance of enzyme purity and specificity in polysaccharide modification and measurement (7). These factors translate directly to dietary fiber (DF) methodology, because the major components of DF are carbohydrate polymers and oligomers. The committee report published in the March issue of Cereal FOODS WORLD refers only to the methodology for measuring enzyme purity and activity (8) that led up the AOAC method 985.29 (2). In this work enzyme purity was gauged by the lack of hydrolysis (i.e., complete recovery) of a particular DF component (e.g. β-glucan, larch galactan or citrus pectin). Enzyme activity was measured by the ability to completely hydrolyze representative starch and protein (namely wheat starch and casein). These requirements and restrictions on enzyme purity and activity were adequate at the time the method was initially developed and served as a useful working guide. However, it was recognized that there was a need for more stringent quality definitions and assay procedures for enzymes used in DF measurements.
Hide AbstractMeasuring dietary fibre.
McCleary, B. V. (1999). The World of Ingredients, 50-53.
Interest in dietary fibre is undergoing a dramatic revival thanks in part to the introduction of new carbohydrates as dietary fibre components. Much emphasis is being placed on determining how much fibre is present in a food. Linking a particular amount of fibre to a specific health benefit is now an important area of research. Total Dietary Fibre. The term “dietary fibre” first appeared in 1953 and referred to hemicelluloses, celluloses and lignin (1). In 1974, Trowell (2) recommended this term as a replacement for the no longer acceptable term “crude fibre” Burkitt (3) has likened the interest in dietary fibre to the growth of a river from its first trickle to a mighty torrent. He observes that dietary fibre “was viewed as merely the less digestible constituent of food which exerts a laxative action by irritating the gut “thus acquiring the designation “roughage” a term which was later replaced by “crude fibre” and ultimately by “dietary fibre” Various definitions of dietary fibre have appeared over the years, partly due the various concepts used in deriving the term (i.e. origin of material, resistance to digestion, fermentation in the colon etc.), and partly to the difficulties associated with its measurement and labelling (4). The principle components of dietary fibre, as traditionally understood, are non-starch polysaccharides, which in plant fibre are principally hemicelluloses and celluloses, and the non-carbohydrate phenolic components, cutin, suberin and waxes with which they are associated in Nature.
Hide AbstractImprovement of rice noodle quality by saturated-steam heat moisture treatment.
Yan, X., Luo, S., Ye, J. & Liu, C. (2025). Carbohydrate Polymers, 123303.
Heat moisture treatment (HMT) of starch granules is a successful technique for enhancing rice noodle quality; however, conventional HMT is time-consuming. In this study, efficient saturated-steam HMT (SS-HMT) was employed for gel modification to enhance rice noodle quality. This treatment was performed under saturated steam (produced under atmospheric pressure in a water bath at 100°C) for brief durations (5, 10, 15, and 20 min), and the underlying mechanism was investigated by examining the variation in starch multiscale structures. SS-HMT disrupted the short double helices and single helices and promoted the formation of longer double helices through rearrangement, increasing the network tie-point size and starch thermal stability. High thermal stability reduced starch leaching and minimized damage to the gas cell walls during cooking, resulting in thicker gas cell walls that enhanced the samples' mechanical strength. SS-HMT markedly improved rice noodle quality. Compared with the control group, rice noodles treated with SS-HMT for 10 min exhibited a 56.05% reduction in cooking loss, a 100% decrease in breakage rate, a 48.46 % increase in hardness, and a 24.68% decrease in adhesiveness. This study provides a straightforward and efficient strategy for improving rice noodle quality.
Hide AbstractImpact of added enzyme-treated bran on the techno-functional properties of puffed-extruded sorghum snack.
Antwi, C. K., Rosa-Sibakov, N. & Emmambux, M. N. (2024). Journal of Cereal Science, 120, 104051.
Dietary fibre intake is crucial for improving human health and reducing the prevalence of diet-related non-communicable diseases. This study determines the effect of the enzyme (Viscozyme®L: a cocktail of cell wall degrading enzymes including arabanase, cellulase, β-glucanase, hemicellulase and xylanase) hydrolyzed fibre on the techno-functional properties of puffed-extruded sorghum snacks made from sorghum flour. Sorghum flour and sorghum flour mixed with untreated bran, water-incubated bran, and enzyme-treated bran were extruded using a twin-screw extruder. The puffed-extruded snacks from sorghum flour with 2-h enzyme-treated bran showed similar expansion ratios to snacks from sorghum flour without bran. The expansion ratios of the without-bran snacks (2.80) and enzyme-treated bran-added snacks (2.77) were significantly (P < 0.05) higher than those made with untreated sorghum bran (1.87). A higher expansion ratio usually results in a lighter, crispier texture and a more attractive appearance of puffed-extruded snacks, which consumers often prefer. The enzyme treatment also increased the water solubility index and decreased the water absorption index compared to snacks with untreated bran. This study explores the potential of using an enzyme-treated sorghum bran to manufacture puffed-extruded snacks comparable to those made with sorghum flour.
Hide AbstractBasic Composition, Antioxidative Properties, and Selected Mineral Content of the Young Shoots of Nigella (Nigella sativa L.), Safflower (Carthamus tinctorius L.), and Camelina (Camelina sativa L.) at Different Stages of Vegetation.
Kapusta-Duch, J., Smoleń, S., Jędrszczyk, E., Leszczyńska, T. & Borczak, B. (2024). Applied Sciences, 14(3), 1065.
Young shoots are a completely new and rapidly growing group of foodstuffs. Also known as “vegetable confetti”, they are a useful addition to commonly consumed meals and often serve a decorative purpose, especially when paired with traditional dishes. Most users are unaware of their invaluable properties as a source of bioactive compounds and add them simply as a dish garnish. Hence, the aim of this study is to evaluate and compare selected health quality parameters of the young shoots of rare oilseed plants (Nigella sativa L., Carthamus tinctorius L., and Camelina sativa L.), which have not been studied in the literature. They are examined for proximate composition (dry matter, total protein, crude fat, ash, digestible carbohydrates, dietary fiber), antioxidative properties (vitamin C, total carotenoids, and total polyphenol content), the content of sixteen selected minerals (calcium, potassium, magnesium, sodium, phosphorus, sulphur, selenium, barium, iron, lithium, beryllium, nickel, gallium, indium, bismuth, silver) as well as antioxidant activity at two harvest dates. The ready-to-eat young shoots in the phase of intensive growth are characterized by a very high content of the examined components and antioxidant properties, which differ depending on the harvest date and plant species. Significantly higher contents of protein, fat, and some minerals have been found in the young shoots from the first harvest compared to those from the second harvest. The antioxidant properties of the young shoots generally increase with maturity. It was not possible, however, to conclusively assess which species of young shoots show the highest health quality.
Hide AbstractPhysicochemical Characteristics and Storage Stability of Hybrid Beef Patty Using Shiitake Mushroom (Lentinus edodes).
Park, G., Oh, S., Park, S., Kim, Y., Park, Y., Kim, Y., Lee, H. & Choi, J. (2023). Journal of Food Quality, 2023, 7239709.
This study evaluated the physicochemical characteristics and storage stability (at 0, 3, and 7 days) of hybrid beef patties with different amount of shiitake mushrooms (Lentinus edodes) added. Shiitake mushrooms contain healthy ingredients such as ergosterol and β-glucan. Four proportions of shiitake mushrooms were added to beef patties (T1, 20%, T2, 40%, T3, 60%, T4, 80%) as a substitute for beef and compared with a control group (CON 0%). Chemical composition, water holding capacity (WHC), cooking loss, pH, color, texture profile analysis, and sensory properties of the products were compared on day 0. As a storage stability experiment, volatile basic nitrogen (VBN), 2-thiobarbituric acid reactive substances (TBARS), and total microbial count were compared (0, 3, and 7 days). The results revealed that replacement with shiitake improved the WHC and cooking loss of patties but had a negative effect on sensory properties and storage stability. These results indicate that shiitake mushrooms can be added along with beef to produce hybrid patties; however, the usage amount must be considered.
Hide AbstractIn Vitro Bioaccessibility of Proteins and Bioactive Compounds of Wild and Cultivated Seaweeds from the Gulf of Saint Lawrence.
Vasconcelos, M. M., Marson, G. V., Rioux, L. E., Tamigneaux, E., Turgeon, S. L., & Beaulieu, L. (2023). Marine Drugs, 21(2), 102.
Despite the increased interest in macroalgae protein and fibers, little information is available on their bioaccessibility. The application of an in vitro gastrointestinal digestion model to study the degree of disintegration and release of proteins with expressed bioactivities from wild and cultivated Palmaria palmata and Saccharina latissima was proposed in this study. Macroalgae from the Gulf of St Lawrence, Canada, were submitted to digestive transit times of 2 (oral), 60 (gastric) and 120 (duodenal) minutes. Among wild samples, P. palmata had a higher percentage of disintegration, protein release and degree of hydrolysis than S. latissima. While the least digested sample, wild S. latissima, was the sample with the highest antioxidant activity (210 μmol TE g−1), the most digested sample, cultivated P. palmata, presented the highest ability to inhibit the angiotensin-converting enzyme (ACE), reaching 32.6 ± 1.2% at 3 mg mL−1. ACE inhibitory activity increased from 1 to 3 mg mL−1, but not at 5 mg mL−1. Wild samples from both species showed an ACE inhibition around 27.5%. Data suggested that the disintegration of the samples was influenced by their soluble and insoluble fiber contents. Further information on the bioaccessibility and bioactivity of these macroalgae should consider the characterization of digestion products other than protein, as well as the effects of previous product processing.
Hide AbstractPhysicochemical and antioxidant properties of rice bran protein hydrolysates obtained from different proteases.
Onsaard, W., Kate-Ngam, S. & Onsaard, E. (2023). Journal of Food Measurement and Characterization, 17(3), 2374-2385.
Rice bran is a by-product obtained from the rice grain milling process that has amounts of nutrients. The objective of this study was to characterize the physicochemical and antioxidant properties of rice bran protein hydrolyzes (RBPHs) by Bacillus licheniformis (RBPH-BL), Aspergillus oryzae (RBPH-AO), and α-chymotrypsin (RBPH-C). The amino acid profile, secondary protein structure, molecular weight, and antioxidant activities of RBPHs were investigated. It was observed that RBPH-C (44%) exhibited a higher degree of hydrolysis (DH) than the other RBPHs, whereas RBPH–BL (134.65 mg/kg) provided the highest content of total essential amino acids. The amino acid composition of the RBPHs compared favorably with the reference standard recommended by WHO/FAO/UNU. The RBPHs were composed of mainly β-sheet secondary structures with a low molecular weight. The RBPHs exhibited high DPPH scavenging ability and reducing power, whereas the RBPH-BL exhibited lipid peroxidation inhibition with an increase in DH. Therefore, RBPH, with its abundant amino acids and high antioxidant activity, can be a potential plant protein source in the food industry.
Hide AbstractEffects of Whole Brown Bean and Its Isolated Fiber Fraction on Plasma Lipid Profile, Atherosclerosis, Gut Microbiota, and Microbiota-Dependent Metabolites in Apoe-/- Mice.
Liu, J., Hefni, M. E., Witthoft, C. M., Bergstrom, M., Burleigh, S., Nyman, M. & Hallenius, F. (2022). Nutrients, 14(5), 937.
The health benefits of bean consumption are widely recognized and are largely attributed to the dietary fiber content. This study investigated and compared the effects of whole brown beans and an isolated bean dietary fiber fraction on the plasma lipid profile, atherosclerotic plaque amount, gut microbiota, and microbiota-dependent metabolites (cecal short-chain fatty acids (SCFAs) and plasma methylamines) in Apoe-/- mice fed high fat diets for 10.5 weeks. The results showed that both whole bean and the isolated fiber fraction had a tendency to lower atherosclerotic plaque amount, but not plasma lipid concentration. The whole bean diet led to a significantly higher diversity of gut microbiota compared with the high fat diet. Both bean diets resulted in a lower Firmicutes/Bacteroidetes ratio, higher relative abundance of unclassified S24-7, Prevotella, Bifidobacterium, and unclassified Clostridiales, and lower abundance of Lactobacillus. Both bean diets resulted in higher formation of all cecal SCFAs (higher proportion of propionic acid and lower proportion of acetic acid) and higher plasma trimethylamine N-oxide concentrations compared with the high fat diet. Whole beans and the isolated fiber fraction exerted similar positive effects on atherosclerotic plaque amount, gut microbiota, and cecal SCFAs in Apoe-/- mice compared with the control diets.
Hide AbstractBioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants.
Cabanillas-Bojórquez, L. A., Gutiérrez-Grijalva, E. P., Castillo-López, R. I., Contreras-Angulo, L. A., Angulo-Escalante, M. A., López-Martínez, L. X., Rios-Iribe, E. Y. & Heredia, J. B. (2021). Fermentation, 7(4), 312.
The production of marine foods is on the rise, and shrimp is one of the most widely consumed. As a result, a considerable amount of shrimp waste is generated, becoming a hazardous problem. Shrimp waste is a rich source of added-value components such as proteins, lipids, chitin, minerals, and carotenoids; however, new bioprocesses are needed to obtain these components. This work aimed to characterize the chemical and nutraceutical constituents from the liquor of shrimp waste recovered during a lactic acid fermentation process using the novel substrate sources whey and molasses. Our results showed that the lyophilized liquor is a rich source of proteins (25.40 ± 0.67%), carbohydrates (38.92 ± 0.19%), minerals (calcium and potassium), saturated fatty acids (palmitic, stearic, myristic and lauric acids), unsaturated fatty acids (oleic acid, linoleic, and palmitoleic acids), and astaxanthin (0.50 ± 0.02 µg astaxanthin/g). Moreover, fermentation is a bioprocess that allowed us to obtain antioxidants such as carotenoids with an antioxidant capacity of 154.43 ± 4.73 µM Trolox equivalent/g evaluated by the ABTS method. Our study showed that liquor from shrimp waste fermentation could be a source of nutraceutical constituents with pharmaceutical applications. However, further studies are needed to separate these added-value components from the liquor matrix.
Hide AbstractRice with Multilayer Aleurone: A Larger Sink for Multiple Micronutrients.
Yu, R., Wu, X., Liu, J., Howitt, C. A., Bird, A. R., Liu, C. M. & Larkin, P. J. (2021). Rice, 14(1), 1-18.
Diet-related noncommunicable diseases impose a heavy burden on human health worldwide. Rice is a good target for diet-related disease prevention strategies because it is widely consumed. Liu et al. demonstrated that increasing the number of cell layers and thickness of putative aleurone in ta2-1 (thick aleurone 2-1) mutant rice enhances simultaneously the content of multiple micronutrients. However, the increases of aleurone-associated nutrients were not proportional to the increases in the aleurone thickness. In this study, first, cytohistological analyses and transmission electron microscopy demonstrated that the multilayer in ta2-1 exhibited aleurone cell structural features. Second, we detected an increase in insoluble fibre and insoluble bound-phenolic compounds, a shift in aleurone-specific neutral non-starch polysaccharide profile, enhancement of phytate and minerals such as iron, zinc, potassium, magnesium, sulphur, and manganese, enrichment of triacylglycerol and phosphatidylcholine but slight reduction in free fatty acid, and an increase in oleic fatty acid composition. These findings support our hypothesis that the expanded aleurone-like layers in ta2-1 maintained some of the distinctive aleurone features and composition. We provide perspectives to achieve even greater filling of this expanded micronutrient sink to provide a means for multiple micronutrient enhancements in rice.
Hide Abstract