| Content: | 1 g or 2 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Below -10oC |
| Formulation: | In BSA plus ammonium sulphate powder |
| Physical Form: | Powder |
| Stability: | > 1 year under recommended storage conditions |
| Enzyme Activity: | β-Amylase |
| EC Number: | 3.2.1.2 |
| CAZy Family: | GH14 |
| CAS Number: | 9000-91-3 |
| Synonyms: | beta-amylase; 4-alpha-D-glucan maltohydrolase |
| Source: | Hordeum vulgare |
| Molecular Weight: | 58,300 |
| Expression: | From barley flour |
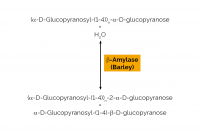
| Specificity: | Hydrolysis of (1,4)-α-D-glucosidic linkages in polysaccharides so as to remove successive maltose units from the non-reducing ends of the chains. |
| Specific Activity: | ~ 500 U/mg (40oC, pH 6.0 on soluble starch) |
| Unit Definition: | One Unit of β-amylase activity is defined as the amount of enzyme required to release one µmole of maltose reducing-sugar equivalents per minute from soluble starch (10 mg/mL) in sodium phosphate buffer (200 mM), pH 6.0 at 40oC. |
| Temperature Optima: | 60oC |
| pH Optima: | 6 |
| Application examples: | For use in AACC and ASBC α-amylase assay procedures. |
| Method recognition: | EBC Method 4.13 and ASBC Method Malt 7 |
The E-BARBP-1G pack size has been discontinued (read more).
High purity β-Amylase (Barley) for use in research, biochemical enzyme assays and analytical testing applications.
Powder enzyme form (2000°L) is for use in AACC and ASBC α-amylase assay procedures.
Data booklets for each pack size are located in the Documents tab.
View our complete list of β-amylase and other Carbohydrate Active enZYme products.
McCleary, B. V. & Codd, R. (1989). Journal of Cereal Science, 9(1), 17-33.
A procedure previously developed for the assay of cereal-flour β-amylase has been improved and standardised. The improved procedure uses the substrate p-nitrophenyl maltopentaose (PNPG5) in the presence of near saturating levels of α-glucosidase. PNPG5 is rapidly hydrolysed by β-amylase but less readily by cereal α-amylases. The substrate is hydrolysed by β-amylase to maltose and p-nitrophenyl maltotriose (PNPG3). With the levels of α-glucosidase used in the substrate mixture, PNPG3 is rapidly cleaved to glucose and p-nitrophenol, whereas PNPG5 is resistant to hydrolysis by the α-glucosidase. The assay procedure has been standardised for several β-amylases and the exact degree of interference by cereal α-amylases determined. The procedure can be readily applied to the selective measurement of β-amylase activity in cereal and malted cereal-flours.
Hide AbstractComparison of molecular structure of oca (Oxalis tuberosa), potato, and maize starches.
Zhu, F. & Cui, R. (2019). Food Chemistry, 296, 116-122.
Oca (Oxalis tuberosa) is an underutilized species and represents a novel starch source. Composition and structure of starches from tubers of two commercial oca varieties grown in New Zealand were compared to those of normal maize and potato starches. The phosphorus content of oca starch was ∼60% of that of potato starch. The amylose content of oca starch (∼21%) was lower than that of maize and potato starches (concanavalin A precipitation method). The fine structure of oca amylopectin was much more similar to that of potato amylopectin than to that of maize amylopectin. Oca amylopectin had a shorter internal chain length and less fingerprint B-chains than potato amylopectin. The two oca starches were structurally and compositionally similar. Oca starch granules had a volume moment mean size of 34.5 μm and B-type polymorph. Comparative analysis suggested that oca starch has the potential to be developed as a novel starch source.
Hide AbstractZhu, F. & Hao, C. (2018). Food Hydrocolloids, 80, 206-211.
New Zealand Maori potatoes (Taewa) represent unique genetic resources for potato quality, though they are much underutilized. In this report, the composition and molecular structure of starches from 5 Maori potato varieties were studied. In particular, the internal unit chain composition of the amylopectins in the form of β-limit dextrins were highlighted. Starches from a commercial modern potato variety and a maize variety with normal amylose contents were employed for comparison. Genetic diversity in the amylose (e.g., 22.6% in Moemoe to 28.6% in Turaekuri) and phosphorus (5.4 mg/100 g in Turaekuri to 7.0 mg/100 g in Kowiniwini) contents as well as the molecule structure of the starches (e.g., external chain length of amylopectin ranged from 13.0 glucosyl residues in Turaekuri to 15.8 glucosyl residues in Karuparera) has been revealed. Maori potato amylopectins have the highest amount of long unit and internal chains and the lowest amount of these chains among amylopectins from different sources. Overall, Maori potato starch appeared to be structurally and compositionally similar to modern potato starch.
Hide AbstractHuang, J., Wei, M., Ren, R., Li, H., Liu, S. & Yang, D. (2017). Carbohydrate Polymers, 163, 324-329.
After combined hydrolysis by α-amylase and β-amylase at room temperature, spherical blocklets in diameters of 27-60 nm were observed on the surface of tapioca starch granules by scanning electron micrography (SEM). Tapioca starch (1%, w/w, db, distilled water) was heated by using a rapid visco analyzer (RVA) in four different programs, then the samples were settled and freeze dried, respectively. The SEM images showed that the blocklets swelled at 52°C; the swollen blocklets deformed to olive shape, and linked by molecular chains, formed bead-like structure at 62°C; they started to merge at 72°C (pasting temperature); then the blocklets fused together and their shapes disappeared completely, and the gel network formed at 95°C. Furthermore, the morphological changes of the blocklets were not simultaneously.
Hide AbstractHan, W., Zhang, B., Li, J., Zhao, S., Niu, M., Jia, C. & Xiong, S. (2017). Food Chemistry, 233, 450-456.
Here we concern the molecular fine structure of intermediate material (IM) fraction in regular maize starch (RMS) and Starpro 40 maize starch (S40). IM had a branching degree and a molar mass (M w ) somewhere between amylopectin (AP) and amylose (AM). Compared with AP, IM had more extra-long (Fr I) and long (Fr II) chains and fb3-chains (degree of polymerization (DP) > 36), with a higher average chain length (CL). Also, IM contained less A-chains but more B-chains (both BS-chains with DP 3-25 and BL-chains with DP ≥ 26), accompanied by longer B- and BL-chains, total internal chains (TICL) and average internal chains (ICL), and a similar average external chain length (ECL). Furthermore, relative to RMS-IM, the IM of S40 (with higher apparent amylose content than RMS) showed increases in relatively-long chains, e.g., Fr II, fb3-chains and BL-chains, but reductions in Mw, relatively-short chains (those with DP 6-12, etc.).
Hide AbstractEspinosa-Ramírez, J., Pérez-Carrillo, E. & Serna-Saldívar, S. O. (2014). Journal of Cereal Science, 60(3), 602-609.
The effect of supplementation of amyloglucosidase or β-amylase during mashing of sorghum and barley beers was studied. The research focused in the increments in maltose and glucose concentrations, and their consumption during 144 h fermentation of lager beers. Barley, red sorghum and white sorghum malt beers, produced with regular or waxy sorghum adjuncts, and supplemented with these exogenous enzymes, were produced. The addition of exogenous enzymes increased up to 20% the total sugar content. Regression lines to describe sugar consumption were obtained (R2 >0.951). Amyloglucosidase treatments had higher glucose contents and up to 4 times higher glucose consumption rates compared with unsupplemented or with β-amylase treatments. The latter treatments had maltose as predominant sugar. Amyloglucosidase treatments had higher ethanol production rates, however the maximum production rate was delayed 24 h, compared with maltose-rich treatments. These worts observed incomplete fermentations and contained higher residual sugars. β-amylase treatments were not significantly different compared with their regular counterparts in terms of sugar and ethanol production. The selection of adequate sorghum cultivars for malt and adjuncts as well as the use of an osmotolerant yeast should be considered when exogenous enzymes are supplemented in brewing.
Hide AbstractRichardson, S., Nilsson, G., Cohen, A., Momcilovic, D., Brinkmalm, G. & Gorton, L. (2003). Analytical Chemistry, 75(23), 6499-6508.
The distribution of substituents along the polymer chain in cationic potato amylopectin starch, modified in solution, granular slurry, or dry state, was investigated. The starch derivatives were successively hydrolyzed by different enzymes, followed by characterization of the hydrolysis products obtained by means of electrospray mass spectrometry (ESI-MS) and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). ESI-MS and MALDI-MS were proved to be appropriate techniques for identification of the substituted hydrolysis products, for which there are no standard compounds available. No highly substituted oligomers were found in the hydrolysates, which was taken as an indication of a more or less homogeneous distribution of cationic groups in the amylopectin molecules. Furthermore, from the results obtained it was suggested that the enzymes cleave glucosidic linkages only between unsubstituted glucose units and, preferentially, linkages in sequences containing more than two adjacent unsubstituted units. The determination of the amount of unsubstituted glucose produced from every successive hydrolysis step revealed slight differences between the different starch samples with respect to the homogeneity of the substitution pattern. Among the three samples under investigation, starch cationized in solution was found to have the most and dry-cationized starch the least homogeneous distribution of substituents.
Hide AbstractNilsson, G. S., Richardson, S., Huber, A., Torto, N., Laurell, T. & Gorton, L. (2001). Carbohydrate Polymers, 46(1), 59-68.
Microdialysis was used for sampling enzyme hydrolysis products of starch hydrolysed with β-amylase, pullulanase, and/or isoamylase, to obtain information about the molecular structure of starch. Starches from waxy, normal, and high amylose maize, and from normal and genetically modified potato (amylose deficient) were used, and also commercial potato amyloses. The hydrolysis products were analysed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Simultaneous sampling and sample clean-up were achieved with microdialysis, thus enabling on-line injection into the liquid chromatographic system. The molecular weight cut-off of the membrane allowed for diffusion of small molecules such as oligosaccharides through the membrane, but hindered large molecules, e.g. enzymes and large polysaccharides, from entering the chromatographic system. With microdialysis sampling, it was possible to investigate the short chain fractions of debranched starch in the presence of amylose without pre-fractionation. The microdialysis–HPAEC-PAD system was also used for determination of the A:B chain ratio and the β-limit value. After β-amylolysis, only liberated maltose diffused through the dialysis membrane, which resulted in on-line sample clean-up from branched β-limit dextrin as well as from the enzyme. The proposed method is fast and easy to handle since clean-up of the hydrolysate is achieved on-line with the chromatographic system.
Hide AbstractRichardson, S., Cohen, A. & Gorton, L. (2001). Journal of Chromatography A, 917(1), 111-121.
The use of high-performance anion-exchange chromatography (HPAEC) with pulsed amperometric detection (PAD) coupled on-line with electrospray mass spectrometry (ESI-MS) for analysis of the substitution pattern in chemically modified starch, has been investigated. In order to characterise the distribution of substitution groups along the polymer chain, hydroxypropylated potato amylopectin starch (HPPAP) was subjected to enzymic hydrolysis, followed by analysis of the degradation products by HPAEC-PAD-MS. When using conventional chromatographic techniques for characterisation of enzymic hydrolysates, standard compounds are required for identification of the hydrolysis products. However, the on-line coupling with ESI-MS allowed identification of all products obtained, substituted as well as unsubstituted, and also of those compounds that co-eluted, without the need for standards. Further, HPAEC-PAD-MS was shown to be useful for analysis of the substitution pattern in modified starch; from results obtained it was suggested that the hydroxypropyl groups were homogeneously distributed in the amylopectin molecule. It was also shown that the starch hydrolysing enzymes were hindered by the hydroxypropyl groups and preferentially cleaved glucosidic linkages between unsubstituted glucose units.
Hide AbstractRichardson, S., Nilsson, G. S., Bergquist, K. E., Gorton, L. & Mischnick, P. (2000). Carbohydrate Research, 328(3), 365-373.
The distribution of substituents in hydroxypropylated potato amylopectin starch (amylose deficient) modified in a slurry of granular starch (HPPAPg) or in a polymer ‘solution’ of dissolved starch (HPPAPs), was investigated. The molar substitution (MS) was determined by three different methods: proton nuclear magnetic resonance (1H NMR) spectroscopy, gas-liquid chromatography (GLC) with mass spectrometry, and a colourimetric method. The MS values obtained by 1H NMR spectroscopy were higher than those obtained by GLC–mass spectrometry analysis and colourimetry. The relative ratio of 2-, 3-, and 6-substitution, as well as un-, mono-, and disubstitution in the anhydroglucose unit (AGU) were determined by GLC–mass spectrometry analysis. Results obtained showed no significant difference in molar distribution of hydroxypropyl groups in the AGU between the two derivatives. For analysis of the distribution pattern along the polymer chain, the starch derivatives were hydrolysed by enzymes with different selectivities. Debranching of the polymers indicated that more substituents were located in close vicinity to branching points in HPPAPg than in HPPAPs. Simultaneous α-amylase and amyloglucosidase hydrolysis of HPPAPg liberated more unsubstituted glucose units than the hydrolysis of HPPAPs, indicating a more heterogeneous distribution of substituents in HPPAPg.
Hide Abstract