| Content: | 200 assays per kit |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
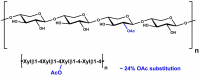
| Analyte: | endo-1,4-β-Xylanase |
| Assay Format: | Spectrophotometer |
| Detection Method: | Absorbance |
| Wavelength (nm): | 590 |
| Signal Response: | Increase |
| Limit of Detection: | 0.02 U/mL of assay solution |
| Reproducibility (%): | ~ 5% |
| Total Assay Time: | ~ 30 min |
| Application examples: | Animal feeds, enzyme preparations, bread improver mixtures and other materials. |
| Method recognition: | Used widely in the feed industry |
The Xylanase (Xylazyme AX) assay kit is used for the measurement of endo-1,4-β-D-xylanase in enzyme preparations, bread improver mixtures and animal feeds. Contains Xylazyme AX Tablets and xylanase enzyme controls (A. niger and Trichoderma longibrachiatum).
Browse our full range of test kits for enzyme activities.

- Very cost effective
- All reagents stable for > 2 years during use
- Only test kit available
- Simple format
- Standard included
Mangan, D., Cornaggia, C., Liadova, A., McCormack, N., Ivory, R., McKie, V. A., Ormerod, A. & McCleary, D. V. (2017). Carbohydrate Research, 445, 14-22.
endo-1,4-β-Xylanase (EC 3.2.1.8) is employed across a broad range of industries including animal feed, brewing, baking, biofuels, detergents and pulp (paper). Despite its importance, a rapid, reliable, reproducible, automatable assay for this enzyme that is based on the use of a chemically defined substrate has not been described to date. Reported herein is a new enzyme coupled assay procedure, termed the XylX6 assay, that employs a novel substrate, namely 4,6-O-(3-ketobutylidene)-4-nitrophenyl-β-45-O-glucosyl-xylopentaoside. The development of the substrate and associated assay is discussed here and the relationship between the activity values obtained with the XylX6 assay versus traditional reducing sugar assays and its specificity and reproducibility were thoroughly investigated.
Hide AbstractAnalysis of feed enzymes.
McCleary, B. V. (2001). “Enzymes in Farm Animal Nutrition”, (M. Bedford and G. Partridge, Eds.), CAB International, pp. 85-107.
Enzymes are added to animal feed to increase its digestibility, to remove anti-nutritional factors, to improve the availability of components, and for environment reasons (Campbell and Bedford, 1992; Walsh et al., 1993). A wide-variety of carbohydrase, protease, phytase and lipase enzymes find use in animal feeds. In monogastric diets, enzyme activity must be sufficiently high to allow for the relatively short transit time. Also, the enzyme employed must be able to resist unfavourable conditions that may be experienced in feed preparation (e.g. extrusion and pelleting) and that exist in the gastrointestinal tract. Measurement of trace levels of enzymes in animal feed mixtures is difficult. Independent of the enzyme studied, many of the problems experienced are similar; namely, low levels of activity, extraction problems inactivation during feed preparation, non-specific binding to other feed components and inhibition by specific feed-derived inhibitors, e.g. specific xylanase inhibitors in wheat flour (Debyser et al., 1999).
Hide AbstractNew developments in the measurement of α-amylase, endo-protease, β-glucanase and β-xylanase.
McCleary, B. V. & Monaghan, D. (2000). “Proceedings of the Second European Symposium on Enzymes in Grain Processing”, (M. Tenkanen, Ed.), VTT Information Service, pp. 31-38.
Over the past 8 years, we have been actively involved in the development of simple and reliable assay procedures, for the measurement of enzymes of interest to the cereals and related industries. In some instances, different procedures have been developed for the measurement of the same enzyme activity (e.g. α-amylase) in a range of different materials (e.g. malt, cereal grains and fungal preparations). The reasons for different procedures may depend on several factors, such as the need for sensitivity, ease of use, robustness of the substrate mixture, or the possibility for automation. In this presentation, we will present information on our most up-to-date procedures for the measurement of α-amylase, endo-protease, β-glucanase and β-xylanase, with special reference to the use of particular assay formats in particular applications.
Hide AbstractOptimising the response.
Acamovic, T. & McCleary, B. V. (1996). Feed Mix, 4, 14-19.
A fine balance exists between enzyme activity and the adverse effects associated with feed processing. Accurate estimation of enzyme activity in the feed is a pre-requisite to optimising the response.
Hide AbstractMeasurement of endo-1,4-β-D-xylanase.
McCleary, B. V. (1992). “Xylans and Xylanases”, (J. Visser, G. Beldman, M. A. Kusters-van Someron and A. G. J. Voragen, Eds.), Progress in Biotechnology, Vol. 7, Elsevier, Science Publishers B. V., pp. 161-169.
Various procedures for the measurement of xylanase in fermentation broths, commercial enzyme mixtures, bread improver mixtures and feed samples are described. Problems associated with the routine use of reducing-sugar based methods axe highlighted and the advantages and limitations of viscometric and dye-labelled substrate procedures for measurement of trace levels of activity in feed samples are discussed.
Hide AbstractMcCleary, B. V. (1991). “Enzymes in Biomass Conversion”, (M. E. Himmel and G. F. Leatham, Eds.), ACS Symposium Series, 460, Chapter 34, pp. 437-449. American Chemical Society, Washington.
Hydrolysis of mannan-type polysaccharides by β-mannanase is dependent on substitution on and within the main-chain as well as the source of the β-mannanase employed. Characterisation of reaction products can be used to define the sub-site binding requirements of the enzymes as well as the fine-structures of the polysaccharides. Action of endo-arabinanase and endo-galactanase on arabinans and arabinogalactans is described. Specific assays for endo-arabinanase and arabinan (in fruit-juice concentrates) are reported.
Hide AbstractMeasurement of polysaccharide degrading enzymes using chromogenic and colorimetric substrates.
McCleary, B. V. (1991). Chemistry in Australia, September, 398-401.
Enzymic degradation of carbohydrates is of major significance in the industrial processing of cereals and fruits. In the production of beer, barley is germinated under well defined conditions (malting) to induce maximum enzyme synthesis with minimum respiration of reserve carbohydrates. The grains are dried and then extracted with water under controlled conditions. The amylolytic enzymes synthesized during malting, as well as those present in the original barley, convert the starch reserves to fermentable sugars. Other enzymes act on the cell wall polysaccharides, mixed-linkage β-glucan and arabinoxylan, reducing the viscosity and thus aiding filtration, and reducing the possibility of subsequent precipitation of polymeric material. In baking, β-amylase and α-amylase give controlled degradation of starch to fermentable sugars so as to sustain yeast growth and gas production. Excess quantities of α-amylase in the flour result in excessive degradation of starch during baking which in turn gives a sticky crumb texture and subsequent problems with bread slicing. Juice yield from fruit pulp is significantly improved if cell-wall degrading enzymes are used to destroy the three-dimensional structure and water binding capacity of the pectic polysaccharide components of the cell walls. Problems of routine and reliable assay of carbohydrate degrading enzymes in the presence of high levels of sugar compounds are experienced with such industrial process.
Hide AbstractWhole-Genome Sequence and Interaction Analysis in the Production of Six Enzymes From the Three Bacillus Strains Present in a Commercial Direct-Fed Microbial (Norum™) Using a Bliss Independence Test.
Hernandez-Patlan, D., Solis-Cruz, B., Latorre, J. D., Merino-Guzman, R., Rodríguez, M. M., Ausland, C., Hernandez-Velasco, X., Holguin, O. R., Delgado, R., Hargis, B. M., Singh, P. & Tellez-Isaias, G. (2022). Frontiers in Veterinary Science, 9.
The three Bacillus strains present in Norum™ were initially selected by their excellent to good relative enzyme activity (REA) production score for amylase, protease, lipase, phytase, cellulase, β-glucanase, and xylanase. Further studies confirmed that the three isolates also showed an antibacterial activity, Gram-positive and Gram-negative poultry pathogens. Norum™ (Eco-Bio/Euxxis Bioscience LLC) is a Bacillus spore direct-fed microbial (DFM). The Bacillus isolates were screened and selected based on in vitro enzyme production profiles. Moreover, in chickens fed high non-starch polysaccharides, this DFM demonstrated to reduce digesta viscosity, bacterial translocation, increase performance, bone mineralization, and balance the intestinal microbiota. In the present study, we present the whole-genome sequence of each of the three isolates in Norum™, as well as the synergistic, additive, or antagonistic effects on the enzyme production behavior of the three Bacillus strains and their combinations when grown together vs. when grown individually. The whole-genome sequence identified isolate AM1002 as Bacillus subtilis (isolate 1), isolate AM0938 as Bacillus amyloliquefaciens (isolate 2), and isolate JD17 as Bacillus licheniformis (isolate 3). The three Bacillus isolates used in the present study produce different enzymes (xylanase, cellulase, phytase, lipase, protease, and β-glucanase). However, this production was modified when two or more Bacillus strains were combined, suggesting possible synergistic, antagonistic, or additive interactions. The Bliss analysis suggested (p < 0.05) that the combination of Bacillus strains 1-2 and 1-2-3 had intermediate effects and predicted that the combination of Bacillus strains 2-3 could have better effects than the combination of all the three Bacillus strains. In summary, the current study demonstrated the need of selecting Bacillus strains based on quantitative enzyme determination and data analysis to assess the impacts of combinations to avoid antagonistic interactions that could limit treatment efficacy. These results suggest that using Bacillus strains 2-3 together could lead to a new generation of DFMs with effects superior to those already examined in Bacillus strains 1-2-3 and, therefore, a potential alternative to growth-promoting antibiotics. More research utilizing poultry models is being considered to confirm and expand the existing findings.
Hide AbstractKim, H. J., Song, Y., Lee, S., Lee, K. P., Lee, B. H. & Yoo, S. H. (2017). Food Research International, 99, 596-602.
Even though the refrigerated dough industry is growing quickly due to the convenience and freshness of refrigerated dough over a prolonged storage period, dough syruping, which is a brownish liquid that leaches out from dough during the storage, is a quality-diminishing factor that needs to be resolved. The objectives of this study were to understand dough syruping and how it is related to structural changes in water-soluble arabinoxylan (WS-AX) and starch in wheat flours during refrigeration as well as to prevent syruping by applying exogenous cell wall polysaccharides. Dough syruping increased to 6.5, 6.9, and 17.2% in weak, strong, and jopoom wheat flours, respectively, after a 35-day storage period. The endoxylanase activity ofjopoom wheat flour was substantially greater compared to other commercial flours, but the activity of this flour did not change over the whole cold storage period. The molecular size reduction of WS-AX was inversely related to the degree of dough syruping. The addition of β-glucan, carboxymethylcellulose, and xylan effectively reduced syrup formation injopoom wheat flour dough.
Hide AbstractMendis, M., Ohm, J. B., Delcour, J. A., Gebruers, K., Meinhardt, S. & Simsek, S. (2013). Cereal Chemistry, 90(3), 240-248.
Arabinoxylans (AX), xylanase, and xylanase inhibitors have an important role in many cereal food processing applications. The effects of genotype, growing location, and their interaction (G × L) on AX, apparent xylanase activity, and apparent xylanase inhibition activity of Triticum aestivum xylanase inhibitor (TAXI) and xylanase inhibiting protein (XIP) were investigated for six hard red and six hard white spring wheat genotypes grown at three locations. Difference in total AX level among genotypes was not determined to a significant level by genotype. Instead, variability in total AX content was largely dependent on G × L. However, total AX content was significantly different between the two wheat classes. For bran xylanase activity, 25% of the variability could be attributed to G × L interaction. Moreover, there was significant difference between the bran xylanase activities in the two wheat classes. Bran TAXI activity and XIP activity were significantly different among genotypes. Genotype contributed 72% to the variability in TAXI activity and 39% in XIP. However, no significant difference was observed among the two wheat classes for TAXI or XIP activity. These results indicate that TAXI might be a stable parameter in segregating wheat genotypes with varying xylanase activity.
Hide AbstractNakamura, S., Satoh, H. & Ohtsubo, K. I. (2011). Journal of Agricultural and Food Chemistry, 59(19), 10665-10676.
As ae mutant rice, such as EM10, lacks the starch branching enzyme IIb, its amylopectin contains more long-chain glucans than that of ordinary Indica and Japonica rice grains. Although boiled grains of ae rice cultivars are too hard and nonsticky for table rice, they are promising in terms of biofunctionality, such as prevention of diabetes. The present paper investigates the characterization of a novel group of four ae mutant rice cultivars (EM72, EM145, EM174, and EM189). They were subjected to the evaluation for their main chemical components, physical properties, and enzyme activities at different grain conditions (raw milled rice, roasted rice, boiled rice, and rice boiled after preroasting). These mutant rice grains are characterized by high apparent amylose, high protein and high glucose contents, high pasting temperature, high α-amylase activities, high resistant starch, and low degree of gelatinization. A novel method was developed to maintain the high resistant starch contents of gelatinized rice grains. Rice boiled after preroasting showed a higher ratio of resistant starch and a lower amount of glucose than ordinary boiled rice. It became possible to produce high-quality and biofunctional pregelatinized rice flours by boiling with frozen fruits, such as tomatoes, after rice grains had been preroasted. These ae mutants were found to be suitable materials for rice/fruit or rice/vegetable products to serve as palatable, low-glucose, and high resistant starch rice products.
Hide AbstractHiller, B., Schlörmann, W., Glei, M. & Lindhauer, M. G. (2011). Food Chemistry, 125(4), 1202-1212.
Seven different types of wheat and rye bread were analysed for colorectal health related compounds, pre and post digestion, in batch fermentation model of the human intestine. Pre digestion, higher amounts of colorectal health-related dietary fibre compounds (soluble/insoluble/total dietary fibre, arabinoxylans, β-glucans) and phytochemicals (mono-/di-phenolic acids, phytic acid, hydroxymethylfurfural) were detected in wholemeal than in refined flour types of bread, as well as in rye flour types than in wheat flour types of bread. Post digestion, faecal bacterial metabolites of colorectal health promoting (acetate/propionate/butyrate, lactate, free mono-/di-phenolic acids) and impairing (amino metabolites, bile acid metabolites) activities were found in fermentation supernatants of bread samples. All types of bread positively affected faecal bacterial metabolism; among the different types of bread, the highest stimulation of organic acid production (acetate/propionate/butyrate, lactate) and the lowest detrimental bacterial enzyme activities (β-glucuronidase, urease) were detected for wheat flour bread, whereas the strongest retardation of bacterial bile acid degradation and the strongest stimulation of phenolic acid metabolite release (phenylpropionic/phenylpropenoic acid derivatives) were induced by wholemeal rye bread. This study for the first time presents a qualitative and quantitative overview over the broad spectrum of colorectal health related compounds in high- and low-fibre types of bread, pre and post in vitro digestion, and highlights the significance of bread for the preventive nutritional intervention of colorectal cancer.
Hide AbstractKabel, M. A., Van der Maarel, M. J. E. C., Klip, G., Voragen, A. G. J. & Schols, H. A. (2006). Biotechnology and Bioengineering, 93(1), 56-63.
Commercial cellulase preparations are potentially effective for processing biomass feedstocks in order to obtain bioethanol. In plant cell walls, cellulose fibrils occur in close association with xylans (monocotyls) or xyloglucans (dicotyls). The enzymatic conversion of cellulose/xylans is a complex process involving the concerted action of exo/endocellulases and cellobiases yielding glucose and xylanases yielding xylooligomers and xylose. An overview of commonly measured cellulase-, cellobiase-, and xylanase-activity, using respectively filter paper, cellobiose, and AZCL-dyed xylan as a substrate of 14 commercially available enzyme preparations from several suppliers is presented. In addition to these standardized tests, the enzyme-efficiency of degrading native substrates was studied. Grass and wheat bran were fractionated into a water unsoluble fraction (WUS), which was free of oligosaccharides and starch. Additionally, cellulose- and xylan-rich fractions were prepared by alkaline extraction of the WUS and were enzymatically digested. Hereby, the capability of cellulose and xylan conversion of the commercial enzyme preparations tested was measured. The results obtained showed that there was a large difference in the performance of the fourteen enzyme samples. Comparing all results, it was concluded that the choice of an enzyme preparation is more dependent on the characteristics of the substrate rather than on standard enzyme-activities measured.
Hide Abstract