| Content: | 2 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 10 years under recommended storage conditions |
| CAS Number: | 9060-75-7 |
| Source: | Sugar-beet pulp |
| Molecular Weight: | 18,000 |
| Purity: | ~ 95% |
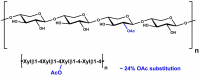
| Monosaccharides (%): | Arabinose: Galactose: Rhamnose = 71: 26: 3 |
| Main Chain Glycosidic Linkage: | α-1,5 |
| Treatment: | Debranched |
| Substrate For (Enzyme): | endo-Arabinanase |
This product has been discontinued (read more).
High purity Debranched Arabinan (Sugar Beet) for use in research, biochemical enzyme assays and in vitro diagnostic analysis.
Browse all available carbohydrates.
McCleary, B. V., McKie, V. A., Draga, A., Rooney, E., Mangan, D. & Larkin, J. (2015). Carbohydrate Research, 407, 79-96.
A range of α-L-arabinofuranosyl-(1-4)-β-D-xylo-oligosaccharides (AXOS) were produced by hydrolysis of wheat flour arabinoxylan (WAX) and acid debranched arabinoxylan (ADWAX), in the presence and absence of an AXH-d3 α-L-arabinofuranosidase, by several GH10 and GH11 β-xylanases. The structures of the oligosaccharides were characterised by GC-MS and NMR and by hydrolysis by a range of α-L-arabinofuranosidases and β-xylosidase. The AXOS were purified and used to characterise the action patterns of the specific α-L-arabinofuranosidases. These enzymes, in combination with either Cellvibrio mixtus or Neocallimastix patriciarum β -xylanase, were used to produce elevated levels of specific AXOS on hydrolysis of WAX, such as 32-α-L-Araf-(1-4)-β-D-xylobiose (A3X), 23-α-L-Araf-(1-4)-β-D-xylotriose (A2XX), 33-α-L-Araf-(1-4)-β-D-xylotriose (A3XX), 22-α-L-Araf-(1-4)-β-D-xylotriose (XA2X), 32-α-L-Araf (1-4)-β-D-xylotriose (XA3X), 23-α-L-Araf-(1-4)-β-D-xylotetraose (XA2XX), 33-α-L-Araf-(1-4)-β-D-xylotetraose (XA3XX), 23 ,33-di-α-L-Araf-(1-4)-β-D-xylotriose (A2+3XX), 23,33-di-α-L-Araf-(1-4)-β-D-xylotetraose (XA2+3XX), 24,34-di-α-L-Araf-(1-4)-β-D-xylopentaose (XA2+3XXX) and 33,34-di-α-L-Araf-(1-4)-β-D-xylopentaose (XA3A3XX), many of which have not previously been produced in sufficient quantities to allow their use as substrates in further enzymic studies. For A2,3XX, yields of approximately 16% of the starting material (wheat arabinoxylan) have been achieved. Mixtures of the α-L-arabinofuranosidases, with specific action on AXOS, have been combined with β-xylosidase and β-xylanase to obtain an optimal mixture for hydrolysis of arabinoxylan to L-arabinose and D-xylose.
Hide AbstractVerhertbruggen, Y., Marcus, S. E., Haeger, A., Verhoef, R., Schols, H. A., McCleary, B. V., McKee, L., Gilbert, H. J. & Knox, J. P. (2009). The Plant Journal, 59(3), 413-425.
Plant cell walls are constructed from a diversity of polysaccharide components. Molecular probes directed to structural elements of these polymers are required to assay polysaccharide structures in situ, and to determine polymer roles in the context of cell wall biology. Here, we report on the isolation and the characterization of three rat monoclonal antibodies that are directed to 1,5-linked arabinans and related polymers. LM13, LM16 and LM17, together with LM6, constitute a set of antibodies that can detect differing aspects of arabinan structures within cell walls. Each of these antibodies binds strongly to isolated sugar beet arabinan samples in ELISAs. Competitive-inhibition ELISAs indicate the antibodies bind differentially to arabinans with the binding of LM6 and LM17 being effectively inhibited by short oligoarabinosides. LM13 binds preferentially to longer oligoarabinosides, and its binding is highly sensitive to arabinanase action, indicating the recognition of a longer linearized arabinan epitope. In contrast, the binding of LM16 to branched arabinan and to cell walls is increased by arabinofuranosidase action. The presence of all epitopes can be differentially modulated in vitro using glycoside hydrolase family 43 and family 51 arabinofuranosidases. In addition, the LM16 epitope is sensitive to the action of β-galactosidase. Immunofluorescence microscopy indicates that the antibodies can be used to detect epitopes in cell walls, and that the four antibodies reveal complex patterns of epitope occurrence that vary between organs and species, and relate both to the probable processing of arabinan structural elements and the differing mechanical properties of cell walls.
Hide AbstractPartial acid-hydrolysis of TEMPO-oxidized arabinoxylans generates arabinoxylan-structure resembling oligosaccharides.
Pandeirada, C. O., Speranza, S., Bakx, E., Westphal, Y., Janssen, H. G. & Schols, H. A. (2021). Carbohydrate Polymers, 275, 118795.
Arabinoxylans (AXs) display biological activities that depend on their chemical structures. To structurally characterize and distinguish AXs using a non-enzymatic approach, various TEMPO-oxidized AXs were partially acid-hydrolysed to obtain diagnostic oligosaccharides (OS). Arabinurono-xylo-oligomer alditols (AUXOS-A) with degree of polymerization 2-5, comprising one and two arabinuronic acid (AraA) substituents were identified in the UHPLC-PGC-MS profiles of three TEMPO-oxidized AXs, namely wheat (ox-WAX), partially-debranched WAX (ox-pD-WAX), and rye (ox-RAX). Characterization of these AUXOS-A highlighted that single-substitution of the Xyl unit preferably occurs at position O-3 for these samples, and that ox-WAX has both more single substituted and more double-substituted xylose residues in its backbone than the other AXs. Characteristic UHPLC-PGC-MS OS profiles, differing in OS abundance and composition, were obtained for each AX. Thus, partial acid-hydrolysis of TEMPO-oxidized AXs with analysis of the released OS by UHPLC-PGC-MS is a promising novel non-enzymatic approach to distinguish AXs and obtain insights into their structures.
Hide AbstractProspection of Fungal Lignocellulolytic Enzymes Produced from Jatoba (Hymenaea courbaril) and Tamarind (Tamarindus indica) Seeds: Scaling for Bioreactor and Saccharification Profile of Sugarcane Bagasse.
Contato, A. G., de Oliveira, T. B., Aranha, G. M., de Freitas, E. N., Vici, A. C., Nogueira, K. M. V., de Lucas, R. C., de Almeida Scarcella, A. S., Buckeridge, M. S., Silva, R. N. & Polizeli, M. D. L. T. D. M. (2021). Microorganisms, 9(3), 533.
The lignocellulosic biomass comprises three main components: cellulose, hemicellulose, and lignin. Degradation and conversion of these three components are attractive to biotechnology. This study aimed to prospect fungal lignocellulolytic enzymes with potential industrial applications, produced through a temporal analysis using Hymenaea courbaril and Tamarindus indica seeds as carbon sources. α-L-arabinofuranosidase, acetyl xylan esterase, endo-1,5-α-L-arabinanase, β-D-galactosidase, β-D-glucosidase, β-glucanase, β-D-xylosidase, cellobiohydrolase, endoglucanase, lichenase, mannanase, polygalacturonase, endo-1,4-β-xylanase, and xyloglucanase activities were determined. The enzymes were produced for eight filamentous fungi: Aspergillus fumigatus, Trametes hirsuta, Lasiodiplodia sp., two strains of Trichoderma longibrachiatum, Neocosmospora perseae, Fusarium sp. and Thermothelomyces thermophilus. The best producers concerning enzymatic activity were T. thermophilus and T. longibrachiatum. The optimal conditions for enzyme production were the media supplemented with tamarind seeds, under agitation, for 72 h. This analysis was essential to demonstrate that cultivation conditions, static and under agitation, exert strong influences on the production of several enzymes produced by different fungi. The kind of sugarcane, pretreatment used, microorganisms, and carbon sources proved limiting sugar profile factors.
Hide AbstractProduction of thermostable endo-1, 5-α-L-arabinanase in Pichia pastoris for enzymatically releasing functional oligosaccharides from sugar beet pulp.
Zhang, N., Wright, T., Wang, X., Savary, B. J. & Xu, J. (2020). Applied Microbiology and Biotechnology, 104(4), 1595-1607.
Sugar beet pulp is an agricultural processing residue that is a rich source of the cell wall polysaccharide arabinan. Functional oligosaccharides, specifically feruloylated arabino-oligosaccharides (FAOs), can be isolated from sugar beet pulp through selective action by endo-arabinanase (glycoside hydrolase family 43). This study aimed to develop yeast (Pichia pastoris) as an efficient, eukaryotic platform to produce a thermophilic endo-1,5-α-L-arabinanase (TS-ABN) for extracting FAOs from sugar beet pulp. Recombinant TS-ABN was secreted into yeast culture medium at a yield of ~ 80 mg/L, and the protein exhibited specific enzyme activity, pH and temperature optimum, and thermostability comparable to those of the native enzyme. Treatment of sugar beet pulp with Pichia-secreted TS-ABN released FAOs recovered by hydrophobic chromatography at 1.52% (w/w). The isolated FAOs averaged seven arabinose residues per ferulic acid, and treatment of T84 human colon epithelial cells significantly increased expression of two key tight junction-related proteins-zonula occludens-1 and occluding-in a dose-dependent manner. This research establishes a biochemical platform for utilizing sugar beet pulp to produce value-added bioproducts with potential nutraceutical applications.
Hide AbstractThe hydrophobic polysaccharides of apple pomace.
Fernandes, P. A., Silva, A. M., Evtuguin, D. V., Nunes, F. M., Wessel, D. F., Cardoso, S. M. & Coimbra, M. A. (2019). Carbohydrate Polymers, 223, 115132.
In this work, polysaccharides extracted with hot water from apple pomace were isolated by C18 cartridge solid-phase extraction at pH 7 (Fr7). Dialysis (12-14 kDa) of this fraction allowed to obtain 17% (w/w) of polymeric material composed by 65% of polysaccharides, mainly arabinose (58 mol%), galacturonic acid (16 mol%) and glucose (10 mol%). Folin-Ciocalteu assay showed 62 g of phloridzin equiv/kg of polyphenols. Moreover, adjusting to pH 3, it was possible to retain an additional fraction (Fr3) representing a further 4% of the polymeric material. Fr3 contained 53% of polysaccharides composed mainly by galacturonic acid (66 mol%) and polyphenols accounted for 37 g of phloridzin equiv/kg. Precipitation with ethanol and subsequent methylation and NMR spectroscopic analysis of Fr7 dialysate allowed the identification of covalently-linked pectic-polyphenol-xyloglucan and arabinan-polyphenol complexes. These structures are possibly formed as a result of polyphenol oxidation reactions during the industrial processing of apples, conferring hydrophobic characteristics to apple pomace polysaccharides.
Hide AbstractPurification and characterization of a new xylanase with excellent stability from Aspergillus flavus and its application in hydrolyzing pretreated corncobs.
Chen, Z., Zaky, A. A., Liu, Y., Chen, Y., Liu, L., Li, S. & Jia, Y. (2019). Protein Expression and Purification, 154, 91-97.
A new extracellular xylanase was purified from a non-toxic mesophilic fungus Aspergillus flavus, and characterized as the β-1, 4-endoxylanase (designated as AfXynA) that appeared in a single protein band on SDS-PAGE with a molecular mass of 20.2 kDa, which is different from all other reported xylanases from the same strain. The AfXynA exhibited a specific activity of 838.2 U/mg. Its optimal temperature and pH were determined to be 55°C and 7.5, respectively. It was stable up to 50°C and within pH 3.5-10.5. AfXynA also exhibited an excellent tolerance to various proteases. This new xylanase had an endohydrolytic mode of action and could hydrolyze xylotriose to xylobiose through transglycosylation. It could efficiently degrade xylan to mainly yield xylobiose, xylotriose, xylopentose and xylohexaose. In addition, the AfXynA was effective in hydrolyzing pretreated corncobs, and shows a great potential in the production of xylooligosaccharides. These unique enzymatic properties make the AfXynA attractive for more biotechnological applications.
Hide AbstractKinetics and regioselectivity of three GH62 α-L-arabinofuranosidases from plant pathogenic fungi.
Sarch, C., Suzuki, H., Master, E. R. & Wang, W. (2019). Biochimica et Biophysica Acta (BBA)-General Subjects, 1863(6), 1070-1078.
Backgound: Xylan is the second most abundant plant cell wall polysaccharide after cellulose with α-L-arabinofuranose (L-Araf) as one of the major side substituents. Capacity to degrade xylan is characteristic of many plant pathogens; and corresponding enzymes that debranch arabinoxylan provide tools to tailor xylan functionality or permit its full hydrolysis. Method: Three GH62_2 family α-arabinofuranosidases (Abfs) from plant pathogenic fungi, NhaAbf62A from Nectria haematococca, SreAbf62A from Sporisorium reilianum and GzeAbf62A from Gibberella zeae, were recombinantly produced in Escherichia coli. Their biochemical properties and substrate specificities were characterized in detail. Particularly with 1H NMR, the regioselectivity and debranching preference of the three Abfs were directly compared. Results: The activities of selected Abfs towards arabinoxylan were all optimal at pH 6.5. Their preferred substrates were wheat arabinoxylan, followed by soluble oat spelt xylan. The Abfs displayed selectivity towards either α-(1 → 2) or α-(1 → 3)-L-Araf mono-substituents in arabinoxylan. Specifically, SreAbf62A and GzeAbf62A removed m-α-(1 → 3)-L-Araf and m-α-(1 → 2)-L-Araf substituents with a similar rates, whereas NhaAbf62A released m-α-(1→ 3)-L-Araf 1.9 times faster than m-α-(1 → 2)-L-Araf. Major conclusions: Building upon the known selectivity of GH62 family α-arabinofuranosidases towards L-Araf mono-substituents in xylans, the current study uncovers enzyme-dependent preferences towards m-α-(1 → 3)-L-Araf and m-α-(1 → 2)-L-Araf substitutions. Comparative sequence-structure analyses of Abfs identified an arginine residue in the xylose binding +2R subsite that was correlated to the observed enzyme-dependent L-Araf debranching preferences. General significance: This study expands the limited pool of characterized GH62 Abfs particularly those from plant pathogenic fungi, and provides biochemical details and methodology to evaluate regioselectivity within this glycoside hydrolase family.
Hide AbstractA novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1.5 L in pretreated corn stover hydrolysis.
Wang, K., Cao, R., Wang, M., Lin, Q., Zhan, R., Xu, H. & Wang, S. (2019). Biotechnology for Biofuels, 12(1), 48.
Background: Cellulose and hemicellulose are the two largest components in lignocellulosic biomass. Enzymes with activities towards cellulose and xylan have attracted great interest in the bioconversion of lignocellulosic biomass, since they have potential in improving the hydrolytic performance and reducing the enzyme costs. Exploring glycoside hydrolases (GHs) with good thermostability and activities on xylan and cellulose would be beneficial to the industrial production of biofuels and bio-based chemicals. Results: A novel GH10 enzyme (XynA) identified from a xylanolytic strain Bacillus sp. KW1 was cloned and expressed. Its optimal pH and temperature were determined to be pH 6.0 and 65°C. Stability analyses revealed that XynA was stable over a broad pH range (pH 6.0-11.0) after being incubated at 25°C for 24 h. Moreover, XynA retained over 95% activity after heat treatment at 60°C for 60 h, and its half-lives at 65°C and 70°C were about 12 h and 1.5 h, respectively. More importantly, in terms of substrate specificity, XynA exhibits hydrolytic activities towards xylans, microcrystalline cellulose (filter paper and Avicel), carboxymethyl cellulose (CMC), cellobiose, p-nitrophenyl-β-D-cellobioside (pNPC), and p-nitrophenyl-β-D-glucopyranoside (pNPG). Furthermore, the addition of XynA into commercial cellulase in the hydrolysis of pretreated corn stover resulted in remarkable increases (the relative increases may up to 90%) in the release of reducing sugars. Finally, it is worth mentioning that XynA only shows high amino acid sequence identity (88%) with rXynAHJ14, a GH10 xylanase with no activity on CMC. The similarities with other characterized GH10 enzymes, including xylanases and bifunctional xylanase/cellulase enzymes, are no more than 30%. Conclusions: XynA is a novel thermostable GH10 xylanase with a wide substrate spectrum. It displays good stability in a broad range of pH and high temperatures, and exhibits activities towards xylans and a wide variety of cellulosic substrates, which are not found in other GH10 enzymes. The enzyme also has high capacity in saccharification of pretreated corn stover. These characteristics make XynA a good candidate not only for assisting cellulase in lignocellulosic biomass hydrolysis, but also for the research on structure-function relationship of bifunctional xylanase/cellulase.
Hide AbstractZhang, J., Zhao, L., Gao, B., Wei, W., Wang, H. & Xie, J. (2018). Journal of Food Processing and Preservation, 42(1), e13367.
Paenibacillus polymyxa Z6 was screened as protopectinase (PPase) producing strain and its PPase activity was 44.4 U/mL. The factors influencing PPase production were identified by a two-level Plackett-Burman design with seven variables. The results indicated that Ca2+ concentration, fermentation time, and temperature were the most influential factors on the PPase production, which were applied in the Box-Behnken design. The predicted maximum PPase activity was 219 U/mL and the experimental maximum PPase activity was 221 U/mL, under the predicted optimum conditions, 170 mg/L Ca2+, 27°C, and 29 hr of fermentation. The present PPase was composed of both type-A PPase, polygalacturonase; and type-B PPase, arabinanase, and rhamnogalacturonase. Finally, the PPase was applied for the pectin extraction from apple pomace and achieved an average yield of 11.9% with properties like 8.5% moisture content, 1.6% ash content, 3.8 mPa.S viscosity, and pH 6.1 of 1% solution.
Hide Abstract