
00:02 Theory of the Analytical Procedure
02:07 Kit Description
03:12 Preparation of Reagent Solutions/Suspensions
05:30 Samples Containing 0–12% Fructan
07:55 Samples Containing 12–100% Fructan
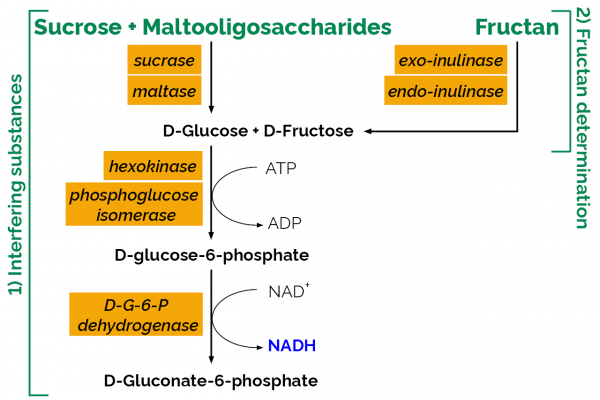
10:07 Analysis of Fructan Content: a. Hydrolysis of Sucrose & Low DP Maltosaccharides
12:39 Analysis of Fructan Content: b. Hydrolysis of Fructan
14:16 Analysis of Fructan Content: c. Measurement of Fructan
17:14 Calculation of Fructan Content
| Content: | 50 assays per kit |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | Fructan |
| Assay Format: | Spectrophotometer |
| Detection Method: | Absorbance |
| Wavelength (nm): | 340 |
| Signal Response: | Increase |
| Linear Range: | 4 to 80 µg of D-glucose, D-fructose or sucrose per assay |
| Limit of Detection: | 1 g/100 g |
| Total Assay Time: | ~ 30 min |
| Application examples: | Flours, plant materials (e.g. onion), food products and other materials |
The Fructan HK test kit is suitable for the specific measurement and analysis of all fructo-oligosaccharides (reducing and non-reducing) and of fructan polysaccharides but is not suitable for the analysis of samples containing high levels of D-glucose, D-fructose, sucrose or maltose.
View our full range of assay kits for polysaccharides.

- Very cost effective
- All reagents stable for > 12 months after preparation
- Fructan kits are available only from Megazyme
- Simple format
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
McCleary, B. V., Charnock, S. J., Rossiter, P. C., O’Shea, M. F., Power, A. M. & Lloyd, R. M. (2006). Journal of the Science of Food and Agriculture, 86(11), 1648-1661.
Procedures for the measurement of starch, starch damage (gelatinised starch), resistant starch and the amylose/amylopectin content of starch, β-glucan, fructan, glucomannan and galactosyl-sucrose oligosaccharides (raffinose, stachyose and verbascose) in plant material, animal feeds and foods are described. Most of these methods have been successfully subjected to interlaboratory evaluation. All methods are based on the use of enzymes either purified by conventional chromatography or produced using molecular biology techniques. Such methods allow specific, accurate and reliable quantification of a particular component. Problems in calculating the actual weight of galactosyl-sucrose oligosaccharides in test samples are discussed in detail.
Hide AbstractMeasurement of total fructan in foods by enzymatic/spectrophotometric method: Collaborative study.
McCleary, B. V., Murphy, A. & Mugford, D. C. (2000). Journal of AOAC International, 83(2), 356-364.
An AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measuring oligofructans and fructan polysaccharide (inulins) in mixed materials and food products. The sample is extracted with hot water, and an aliquot is treated with a mixture of sucrase (a specific sucrose-degrading enzyme), α-amylase, pullulanase, and maltase to hydrolyze sucrose to glucose and fructose, and starch to glucose. These reducing sugars are then reduced to sugar alcohols by treatment with alkaline borohydride solution. The solution is neutralized, and excess borohydride is removed with dilute acetic acid. The fructan is hydrolyzed to fructose and glucose using a mixture of purified exo- and endo-inulinanases (fructanase mixture). The reducing sugars produced (fructose and glucose) are measured with a spectrophotometer after reaction with para-hydroxybenzoic acid hydrazide. The samples analyzed included pure fructan, chocolate, low-fat spread, milk powder, vitamin tablets, onion powder, Jerusalem artichoke flour, wheat stalks, and a sucrose/cellulose control flour. Repeatability relative standard deviations ranged from 2.3 to 7.3%; reproducibility relative standard deviations ranged from 5.0 to 10.8%.
Hide AbstractMeasurement of inulin and oligofructan.
McCleary, B. V. & Blakeney, A. B. (1999). Cereal Foods World, 44, 398-406.
Fructans are defined as any compound in which one or more fructosyl-fructose linkages constitute a majority of the linkages (1). This refers to polymeric material as well as to oligomers as small as disaccharide inulobiose. Fructans are widely distributed in the plant kingdom. They are present in monocotyledons, dicotyledons, and green algae. Fructans differ in molecular structure and in molecular weight. They may be classified into three main types, the inulin type, the levan (previously called phlein) type, and the graminan type (2). The inulin group consists of material that has mostly of exclusively the (2-1) fructosly-fructose linkage. Levan is material that contains mostly or exclusively the (2-6) fructosyl-fructose linkage. The graminan (or branched) type has both (2-1) and (2-6) fructosly-fructose linkages in significant amounts (e.g. graminan from Gramineae). The distribution of fructans in nature, and the production of fructooligosaccharides, such as neosugar, using fructosyltransferase, has been reviewed in a monograph (3). In the context of this article and the analytical procedure described, the term fructan relates only to inulin and graminan. The current analytical procedure has not been evaluated on levan.
Hide AbstractMeasurement of inulin and inulin-degrading enzymes.
McCleary, B. V. (1998). “Proceedings of the Seventh Seminar on Inulin”, (A. Fuchs and A. Van Laere, Eds.), European Fructan Association, pp. 36-45.
A non-instrumental method for the measurement of fructan is described. The method simplifies fructan analysis, is easy to perform, uses standard laboratory equipment, and is accurate, reproducible and specific. The procedure employs highly purified and specific enzymes to hydrolyse sucrose, starch and fructans (inulins and graminan).
Hide AbstractFructans - Analytical approaches to a fibre that ferments.
Blakeney, A. B., McCleary, B. V. & Mugford, D. C. (1997). Chemistry in Australia, 17-19.
Fructans are defined as any compound where one or more fructosyl-fructose linkages constitute a majority of the linkages. This refers to polymeric material as well as oligomers as small as the diasaccharide inulobiose. Material included in this definition may or may not contain attached glucose. The terms oligomer and polymer are used by fructan researchers to distinguish between materials that can be specifically characterised and those that can not. Fructans are widely distributed in the plant kingdom. They are present in monocotyledons, dicotyledons and in green algae.
Hide AbstractMcCleary, B. V., Gibson, T. S. & Mugford, D. C. (1997). Journal of AOAC International, 80, 571-579.
An American Association of Cereal Chemists/AOAC collaborative study was conducted to evaluate the accuracy and reliability of an enzyme assay kit procedure for measurement of total starch in a range of cereal grains and products. The flour sample is incubated at 95 degrees C with thermostable alpha-amylase to catalyze the hydrolysis of starch to maltodextrins, the pH of the slurry is adjusted, and the slurry is treated with a highly purified amyloglucosidase to quantitatively hydrolyze the dextrins to glucose. Glucose is measured with glucose oxidase-peroxidase reagent. Thirty-two collaborators were sent 16 homogeneous test samples as 8 blind duplicates. These samples included chicken feed pellets, white bread, green peas, high-amylose maize starch, white wheat flour, wheat starch, oat bran, and spaghetti. All samples were analyzed by the standard procedure as detailed above; 4 samples (high-amylose maize starch and wheat starch) were also analyzed by a method that requires the samples to be cooked first in dimethyl sulfoxide (DMSO). Relative standard deviations for repeatability (RSD(r)) ranged from 2.1 to 3.9%, and relative standard deviations for reproducibility (RSD(R)) ranged from 2.9 to 5.7%. The RSD(R) value for high amylose maize starch analyzed by the standard (non-DMSO) procedure was 5.7%; the value was reduced to 2.9% when the DMSO procedure was used, and the determined starch values increased from 86.9 to 97.2%.
Hide AbstractMcCleary, B. V. (1994). “Methods in Carbohydrate Chemistry”, Vol. X, (J. N. BeMiller, D. J. Manners and R. J. Sturgeon, Eds.), John Wiley & Sons Inc., pp. 175-182.
A number of methods have been described for the analysis of the fine structure of galactomannans, i.e., the distribution of D-galactosyl units along the D-mannan backbone (1). Such studies include the analysis of x-ray diffraction data of stretched fibers of galactomannans (2,3), 1H- and 13C-nmr (nuclear magnetic resonance) of native and partially depolymerized galacto¬mannans (4) and a range of chemical procedures (5-7), including those employing a detailed theoretical analysis of the kinetics of reaction (8). An alternative approach involves the characterization and quantification of the oligosaccharides produced on hydrolysis of galactomannans by highly purified and well-characterized β-mannanases (EC 3.2.1.78) (9,10). The β-mannanases employed were purified to homogeneity by affinity chromatography on gIucornannan-AH-Sepharose 4B. They were characterized by a range of physicochemicai procedures by determining the kinetics of their action on β-mannooligosaccharides, and by characterizing the structures of oligosaccharides produced on hydrolysis of galactomannans and glucomannans (11). From these studies, a basic model describing the subsite binding requirements of all the β-mannanases examined was proposed (Fig. 1). This model was then modified to account for the slight differences noted in the types of oligosaccharides produced by β-mannanases from different sources. The β-mannanases which differ most significantly in their action patterns on galactomannans are those from Aspergillus niger culture filtrates and from germinated guar seed.
Hide AbstractAgro-Residues and Sucrose Alternatives in Confectionery Transformation Towards Glucose Spikes Minimization.
Zlatanović, S., Laličić-Petronijević, J., Pastor, F., Micić, D., Dodevska, M., Stevanović, M., Karlovic, S. & Gorjanović, S. (2025). Foods, 14(3), 491.
Apple and beetroot pomace flour (APF and BPF), along with two sweeteners, sucrose and a blend of sucrose substitutes (erythritol, stevia, inulin, and fructose), were simultaneously incorporated into three matrices formulated with agar, pectin, or gelatin as gelling agents. The aim was to produce jelly candies with high content of dietary fiber and dietary phenolics, and reduced energy value. The simultaneous incorporation of sucrose substitutes and pomace flour resulted in decrease of Carb:Fiber and Sugar:Fiber Ratio to extremely low values of 2.7-3.4 and 1.3-1.6 respectively, as well as in Energy:Fiber Ratio decrease to 9.2-12.3 kcal/g DF. Relative Antioxidant Capacity Index (RACI), as indicator of antioxidant potential, was calculated by assigning equal weight to Folin-Ciocâlteu, DPPH and FRAP assays applied upon in vitro digestion of 18 formulations of jelly candies. Results obtained for formulations with and without sucrose, as well as with and without APF or BPF, enabled insight into effects of pomace flour addition and sucrose substitution in each gelling matrix on functional properties. The incorporation and the substitution impact on postprandial glucose response were followed in vivo. Their superimposing resulted in glycemic index below 30 and low glycemic load. Efficiency of applied approach in functionalization of confectionery burden with energy and minimization of glucose spike represent an example of agro-residues re-introduction with the highest potential contribution to anti-obesity strategy.
Hide AbstractInter-and intraspecific study on compositional quality traits of diverse sets of spelt and common wheat.
Rakszegi, M., Tóth, V., Tömösközi, S., Jaksics, E., Cseh, A., Karsai, I. & Mikó, P. (2025). Journal of Cereal Science, 123, 104162.
Genetically diverse fifty spelt and fifty bread wheat genotypes selected based on the results of a 25K SNP array analysis were compared for physical, compositional and breadmaking quality traits. Inter- and intraspecific diversity, the effect of the species, the genotypes and the year was evaluated. The variation found in the compositional and processing traits of spelt was narrower than that of bread wheat. Significant effect of the genotype was identified for both species for almost all the traits, and spelt genotypes were found to have longer kernels, higher protein content, and lower starch, fructan and β-glucan content than bread wheat in average. Processing of spelt was determined by the more extensible dough, lower starch damage and water absorption of the flour, the low starch viscosity values, high pasting-time and temperature. Although spelt comes with challenges in bread-making quality due to weaker gluten properties, its potential in functional food applications, specialized diets and organic cultivation is promising.
Hide AbstractExploitation of cardoon roots inulin for polyhydroxyalkanoate production.
Corrado, I., Borselleca, E., Dal Poggetto, G., Staiano, I., Alfieri, M. L. & Pezzella, C. (2024). Industrial Crops and Products, 214, 118570.
Cynara cardunculus, cardoon, is a biorefinery crop with an overwhelming role in the bioplastic scenario. This work explored the use of inulin extracted from cardoon roots as a feedstock for polyhydroxyalkanoates (PHA) production. Cardoon roots from both spring and winter seasons were subjected to two protocols consisting of an autoclave extraction followed by i) an ethanol precipitation and further lyophilization or ii) lyophilization directly. The resulting extracts were characterized for recovery yield (from 11.6 to 16 g of inulin per 100 g of roots), purity grade (from 64% to 97%) and molecular weight distribution, the latter being affected by both seasonal variability and the extraction method. The performances of two PHA producers, Cupriavidus necator and Burkholderia cepacia, were compared in Simultaneous Saccharification and Fermentation of spring inulin extracts obtained with the two protocols, exploring the effect of controlled addition of fungal inulinase PlaI. Up to 2 g/L of polyhydoxybutyrate (PHB) polymer was produced in the best feeding condition, with both strains found able to metabolize the main phenolic acids coextracted with inulin. Diversity in polymer yields were observed, with evidence of the synthesis of PHB polymers characterized by different molecular weight distributions depending on the type of feeding and microorganism employed. The proposed processes are placed in the frame of the circular economy approaches applied to the valorization of cardoon biomass in the bioplastic field.
Hide AbstractEnhancing composition and functionality of jelly candies through apple and beetroot pomace flour addition.
Gorjanović, S., Zlatanović, S., Laličić-Petronijević, J., Dodevska, M., Micić, D., Stevanović, M. & Pastor, F. (2024). npj Science of Food, 8(1), 85.
The functionalization of food products with agri-industial residues is of great interest. Apple and beetroot pomace flour, abundant in dietary fiber and antioxidants, were incorporated into jelly candies using agar, pectin, or gelatin. Three functional formulations were devised for each flour type at the pilot scale, resulting in jelly candies with desirable sensory properties and texture. The high content of total polyphenolics, flavonoids, betacyanins, and betaxantines was determined upon in vitro digestion. The influence of different matrices on these bioactives, crucial for exerting antioxidant activity, was evaluated using DPPH and FRAP assays on both fresh and nine-month stored jelly candies, showcasing good bioavailability and retention. Enrichment with APF and BPF also led to reduced postprandial glucose levels, glycemic index, and load determined in vivo. These findings affirm that compositionally optimized innovative formulations of jelly candies facilitate the efficient delivery of compounds with anti-obesity effect from upcycled raw materials.
Hide AbstractIntegrated enzymatic hydrolysis of crude red onion extract and yeast treatment for production and purification of short-chain inulin and inulin neoseries oligosaccharides.
Wongsanittayarak, J., Leangnim, N., Unban, K., Khanongnuch, C., Lumyong, S., Wongputtisin, P. & Kanpiengjai, A. (2024). Journal of Agriculture and Food Research, 18, 101353.
As has been confirmed in our previous study, red onion is a promising source of inulin-fructan, inulin neoseries fructan, and their fructooligosaccharides (FOSs). Short-chain FOSs (SCFOSs) are potential prebiotics as they provide many health promoting benefits. However, short-chain inulin neoseries oligosaccharides isolated from plants have scarcely been studied. After prebiotic production, a purification step is essential to produce a functional prebiotic. The aim of this study was to develop new processes of integrated enzymatic hydrolysis of crude red onion extract and yeast treatment for production and purification of SCFOSs. Endo-inulinase is a key enzyme for hydrolysis of crude red onion extract to produce SCFOSs, while Candida orthopsilosis FLA44.2 was employed for purification of the produced SCFOSs. Three different strategies, including two-step (TSP), simultaneous (SP), and semi-simultaneous (SSP) production processes, were designed and investigated as potentially simple, cost-effective, and timesaving production processes. The SP and SSP processes met the objective criteria by exhibiting desirable yields of SCFOSs and the optimal purity of SCFOSs when compared with the TSP process. Notably, the SP process was highlighted as it was simpler than the SSP process. Under optimal conditions (0.4 U of endo-inulinase/g total fructans, 5 % v/v of yeast inoculum and reaction time of 72 h), the SP process conducted in 1-dm3 flasks produced SCFOSs of 87 g/dm3 with neo-GF2 as the main constituent (42 g/dm3) and a purity of 100 %. The ratio of total SCFOSs to total fructans was 0.93 indicating that fructans of the red onion extract have been transformed to SCFOSs.
Hide AbstractRheological properties and characterisation of some bioactive components in flours made of different coloured sweet potato (Ipomoea batatas L.) genotypes.
Mihály-Langó, B., Ács, K., Berényi, A., Maróti Tóth, K., Táborosi Ábrahám, Z., Gáll, T. & Ács, E. (2023). Acta Alimentaria, 52(4), 570-578.
The popularity of sweet potatoes in Central Europe has been increasing recently, mainly the high-quality, perfect, fresh tubers are in demand. However, out of class grade tubers could be marketed in dried, grounded form as sweet potato flour. The aim of this study was to characterise some important nutritional properties of flours of three sweet potato genotypes with different tuber colours (white, purple, and orange) and to investigate how this raw material affects the rheological properties of sweet potato-wheat flour blends. Dietary fibres are present in sweet potatoes in a significant proportion, orange coloured flour showed the highest values. The main free sugars were sucrose, glucose, and fructose, but sucrose was the dominant one. Antioxidant capacity and total phenolic content also varied considerably, the purple flour had the highest values. Mineral composition showed significant variability, the purple flour contained the highest level of minerals. It was confirmed that adding sweet potato flour to wheat flour affected its rheological properties, however in a varied manner. For the orange flour these properties have lightly decreased, though it had no significant effect on dough quality, while the white and purple flours with a dosage of 5, 10 and 15% could improve the dough behaviour. Thus, sweet potato in this form is a valuable raw material.
Hide AbstractAlteration of Carbohydrate Metabolism in Fusarium Infected Wheat Kernels Treated with Fungicides and Its Relation to Baking Technological Parameters and Deoxynivalenol Contamination.
Acs, K., Varga, M., Szekeres, A., Salgo, A., Lantos, C., Bekes, F., Pauk, J. & Mesterhazy, A. (2023). Agriculture, 13(4), 868.
Changes of water-soluble carbohydrate (WSC) content such as fructose, glucose, sucrose, maltose, nystose, raffinose, stachyose and fructan were analyzed in wheat kernels in Fusarium epidemic and non-epidemic seasons. In both season types, eight commercial fungicides were applied and three wheat varieties with differing Fusarium resistance were tested. In the epidemic year, the average total amount of WSC was above 1.6% which was 2 times higher than in the non-epidemic year (0.7%). Sucrose, maltose, raffinose and fructan components determined the increased WSC value, but the most substantial change was observed in maltose content where its average amount was 28 times higher in the epidemic year. Fungicide application also significantly increased all the carbohydrate components except maltose, where significant reduction was observed. WSC components had strong correlation with several farinograph or extensograph parameters, but only the maltose content showed positive strong correlation (r = 0.9) with deoxynivalenol (DON) toxin that was highly affected by the applied fungicide. The changes of WSC indicate altered carbohydrate synthesis along with abnormal degradation processes and thus have impaction on the baking features. It seems that the sugar metabolism interacts with DON synthesis and the results give important additional information to the altered metabolism of the attacked plant.
Hide AbstractFibre and short-chain carbohydrate composition in rye varieties, novel industrial milling fractions and breads.
Szentmiklóssy, M. K. J., Jaksics, E., Farkas, A., Pusztai, É., Kemény, S., Németh, R. & Tömösközi, S. (2023). Acta Alimentaria, 52(2), 177-189.
Rye is an important raw material of bread due to tradition and its favourable nutritional and technological qualities. Despite the beneficial fibre composition, a special group of short-chain carbohydrates, the so called FODMAPs (fermentable oligo-, di-, monosaccharides and polyols) may cause problems for patients with irritable bowel syndrome. The aim of our work was to investigate the non-starch carbohydrate (dietary fibre compounds, short-chain carbohydrates) composition of rye varieties, and of their novel milling fractions obtained from industrial milling trials and test loaves made from them. Regarding fibre and short chain carbohydrate composition, rye varieties did not show significant differences. In new subfractions, fibre and FODMAP composition were described, among profiles most of them differ from commonly used flours, independently from variety. The yeast fermentation and baking caused a decrease in water-extractable arabinoxylan content, at the same time increased the substitution pattern of water-extractable arabinoxylans. Furthermore, breadmaking process decreased the fructan content, and therefore increased the fructose level, thus modifying the short-chain carbohydrate composition. Based on our knowledge, this research is among the first ones investigating the fibre and short-chain composition of rye from the seeds to the consumable final products.
Hide Abstract




















