| Content: | 4 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 2 years under recommended storage conditions |
| CAS Number: | 11078-31-2 |
| Source: | Konjac tubers |
| Purity: | > 98% |
| Viscosity: | ~ 10 cSt |
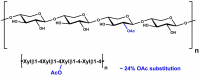
| Monosaccharides (%): | Mannose: Glucose = 60: 40 |
| Main Chain Glycosidic Linkage: | β-1,4 |
| Substrate For (Enzyme): | endo-1,4-β-Glucanase, endo-Cellulase |
High purity Glucomannan (Konjac; Low Viscosity) for use in research, biochemical enzyme assays and analytical testing applications.
Browse all available polysaccharides.
Structural and functional insights into extreme thermal stability and activity of two GH 12 domains of a multidomain glycosidase from a hyperthermophilic euryarchaeon.
Zayulina, K. S., Frolov, E. N., Stracke, C., Klyukina, A. A., Khusnutdinova, A. N., Stogios, P., Skarina, T., Yakunin, A., Golyshin, P. N., Siebers, B., Shugaeva, T. E. & Kublanov, I. V. (2025). The FEBS Journal, 70095.
Bacteria and fungi are well known for efficient degradation of plant polysaccharides thanks to various enzymes involved in plant cell wall decomposition. However, little is known about the role of archaea in this process or the repertoire and features of their polysaccharide-degrading enzymes. In our previous work, we discovered an archaeal multidomain glycosidase (MDG) composed of three catalytic domains (GH5 and two GH12) and two cellulose-binding modules (CBM2). The recombinant MDG and individual GH5 catalytic domain were active against cellulose and a number of other polysaccharides at a wide range of temperatures, with optimum temperatures (Topt) of 60°C and 80°C, respectively. The present study was focused on the characterization of two GH12 domains of the MDG. Purified recombinant TMDG_GH12-1 and TMDG_GH12-2 proteins were active as individual enzymes but exhibited distinct catalytic properties. Both enzymes were thermostable and active at extremely high temperatures: TMDG_GH12-1 was active at 40-130°C (Topt 100°C), and its half-life (t½) at 100°C was 42 h, which makes it one of the most thermostable glycosidases known so far, whereas TMDG_GH12-2 was active at 50-100°C (Topt 90°C) with t½ at 100°C being 30 min. Phylogenetic and structural analysis of both TMDG_GH12 proteins together with molecular docking and site-directed mutagenesis suggested that the presence of two disulfide bridges and the W → Q mutation in the active site contribute to the exceptional thermostability of TMDG_GH12-1. Further structural and mutational studies of the TMDG_GH12-1 domain will help to gain a better understanding of the molecular mechanisms of its extraordinary thermostability and substrate specificity.
Hide AbstractSynergistic effects of distinct arabinofuranosidase specificities in lignocellulose degradation by different hemicellulases.
Pentari, C., Mylona, E. P., Zerva, A. & Topakas, E. (2025). International Journal of Biological Macromolecules, 302, 140575.
Arabinoxylan is a prevalent hemicellulose type, notably heterogeneous and resistant to biodegradation. Arabinofuranosidases (Abfs) remove arabinofuranosyl decorations of arabinoxylan, thus enabling hydrolysis by xylanases. However, a variety of Abf and xylanase specificities have emerged in recent years, necessitating a deeper understanding of their role in biomass degradation. This work investigates the biochemical features of TtAbf43 from Thermothelomyces thermophila, which specifically removes the O-3-linked arabinofuranose moieties from di-substituted xylopyranoses. The enzyme also exhibited secondary hydrolytic activity on o-nitrophenyl-β-d-xylopyranoside and arabinan. The hydrolysis of pretreated wheat and corn bran substrates was assessed using TtAbf43 and AnAbf51, two enzymes with distinct catalytic specificities. The Abfs enhanced the performance of endo-xylanases TmXyn10 and AnXyn11, promoting the release of xylo-oligomers, while the xylanases, in turn, stimulated arabinose release by the Abfs. Additionally, the Abfs facilitated the endo- and exo-activities of the bifunctional xylobiohydrolase/glucuronoxylanase TtXyn30A for the release of xylobiose and short aldouronic acids from biomass. AnAbf51 also acted in synergy with the acetyl xylan esterase OCE6 and the exo-deacetylase TtCE16B in debranching enzymatically derived oligomers from lignocellulose, whereas TtAbf43 remained unaffected by the esterases. These diverse synergistic relationships among different hemicellulases could assist the development of new enzymatic approaches for efficient biomass valorization.
Hide AbstractTranscriptional delineation of polysaccharide utilization loci in the human gut commensal Segatella copri DSM18205 and co-culture with exemplar Bacteroides species on dietary plant glycans.
Panwar, D., Briggs, J., Fraser, A. S., Stewart, W. A. & Brumer, H. (2024). Applied and Environmental Microbiology, e01759-24.
There is growing interest in members of the genus Segatella (family Prevotellaceae) as members of a well-balanced human gut microbiota (HGM). Segatella are particularly associated with the consumption of a diet rich in plant polysaccharides comprising dietary fiber. However, understanding of the molecular basis of complex carbohydrate utilization in Segatella species is currently incomplete. Here, we used RNA sequencing (RNA-seq) of the type strain Segatella copri DSM 18205 (previously Prevotella copri CB7) to define precisely individual polysaccharide utilization loci (PULs) and associated carbohydrate-active enzymes (CAZymes) that are implicated in the catabolism of common fruit, vegetable, and grain polysaccharides (viz. mixed-linkage β-glucans, xyloglucans, xylans, pectins, and inulin). Although many commonalities were observed, several of these systems exhibited significant compositional and organizational differences vis-à-vis homologs in the better-studied Bacteroides (sister family Bacteroidaceae), which predominate in post-industrial HGM. Growth on β-mannans, β(1, 3)-galactans, and microbial β(1, 3)-glucans was not observed, due to an apparent lack of cognate PULs. Most notably, S. copri is unable to grow on starch, due to an incomplete starch utilization system (Sus). Subsequent transcriptional profiling of bellwether Ton-B-dependent transporter-encoding genes revealed that PUL upregulation is rapid and general upon transfer from glucose to plant polysaccharides, reflective of de-repression enabling substrate sensing. Distinct from previous observations of Bacteroides species, we were unable to observe clearly delineated substrate prioritization on a polysaccharide mixture designed to mimic in vitro diverse plant cell wall digesta. Finally, co-culture experiments generally indicated stable co-existence and lack of exclusive competition between S. copri and representative HGM Bacteroides species (Bacteroides thetaiotaomicron and Bacteroides ovatus) on individual polysaccharides, except in cases where corresponding PULs were obviously lacking.
Hide AbstractThe effect of hemicelluloses on biosynthesis, structure and mechanical performance of bacterial cellulose-hemicellulose hydrogels.
Chibrikov, V., Pieczywek, P. M., Cybulska, J. & Zdunek, A. (2024). Scientific Reports, 14(1), 21671.
The primary plant cell wall (PCW) is a specialized structure composed predominantly of cellulose, hemicelluloses and pectin. While the role of cellulose and hemicelluloses in the formation of the PCW scaffold is undeniable, the mechanisms of how hemicelluloses determine the mechanical properties of PCW remain debatable. Thus, we produced bacterial cellulose–hemicellulose hydrogels as PCW analogues, incorporated with hemicelluloses. Next, we treated samples with hemicellulose degrading enzymes, and explored its structural and mechanical properties. As suggested, difference of hemicelluloses in structure and chemical composition resulted in a variety of the properties studied. By analyzing all the direct and indirect evidences we have found that glucomannan, xyloglucan and arabinoxylan increased the width of cellulose fibers both by hemicellulose surface deposition and fiber entrapment. Arabinoxylan increased stresses and moduli of the hydrogel by its reinforcing effect, while for xylan, increase in mechanical properties was determined by establishment of stiff cellulose–cellulose junctions. In contrast, increasing content of xyloglucan decreased stresses and moduli of hydrogel by its weak interactions with cellulose, while glucomannan altered cellulose network formation via surface deposition, decreasing its strength. The current results provide evidence for structure–dependent mechanisms of cellulose–hemicellulose interactions, suggesting the specific structural role of the latter.
Hide AbstractUnravelling the complexity of hemicellulose pyrolysis: Quantitative and detailed product speciation for xylan and glucomannan in TGA and fixed bed reactor.
Piazza, V., da Silva Junior, R. B., Frassoldati, A., Lietti, L., Gambaro, C., Rajendran, K., Chen, D., Faravelli, T. & Beretta, A. (2024). Chemical Engineering Journal, 497, 154579.
For the first time, the complete characterization of the pyrolysis of key biomass components – xylan (representative of pentose-based hardwood hemicellulose) and glucomannan (representative of hexose-based softwood hemicellulose) – is herein obtained by applying a novel methodology based on TGA (thermogravimetric analysis), previously developed for cellulose. Data on devolatilization rates, onset temperatures and detailed quantification of the mass yield of ~ 50 products in the gas, liquid, solid phase were achieved simultaneously, with closure of mass balance, thus providing unique kinetic information. Pyrolysis tests were also carried out in a fixed bed reactor to explore larger scales and validate the TGA-based approach. At both scales, combinations of different analytical techniques (online MS, offline GC-FID/MS, Karl Fischer titration) and sampling protocols (cold condenser, sorbent traps, vapour syringes, gas bags) were tuned to achieve closure of mass balances and rigorous product speciation. While the pyrolysis of cellulose – chosen as reference system − maximized the bio-oil production (mostly levoglucosan), the pyrolysis of xylan resulted into an even distribution among solid, liquid and gas phases, with condensable oxygenates spanning homogeneously in the C1-C9 range. Interestingly, glucomannan showed an intermediate behaviour between cellulose and xylan, mirroring its intermediate chemical structure. Raman and oxidation analyses on the collected char samples showed that the solid residuals from hemicelluloses were less ordered and richer in ashes than those from cellulose. The predictions of recent lumped kinetic models were used to benchmark the new datasets against the prior art on hemicellulose pyrolysis; the richness and comprehensiveness of the new information clearly emerge and pave the pathway to the de-bottlenecking of kinetic modelling.
Hide AbstractCharacterization of cellulases from softening fruit for enzymatic depolymerization of cellulose.
Edema, H., Ashraf, M. F., Samkumar, A., Jaakola, L. & Karppinen, K. (2024). Carbohydrate Polymers, 343, 122493.
Cellulose is a major renewable resource for a wide variety of sustainable industrial products. However, for its utilization, finding new efficient enzymes for plant cell wall depolymerization is crucial. In addition to microbial sources, cellulases also exist in plants, however, are less studied. Fleshy fruit ripening includes enzymatic cell wall hydrolysis, leading to tissue softening. Therefore, bilberry (Vaccinium myrtillus L.), which produces small fruits that undergo extensive and rapid softening, was selected to explore cellulases of plant origin. We identified 20 glycoside hydrolase family 9 (GH9) cellulases from a recently sequenced bilberry genome, including four of which showed fruit ripening-specific expression and could be associated with fruit softening based on phylogenetic, transcriptomic and gene expression analyses. These four cellulases were secreted enzymes: two B-types and two C-types with a carbohydrate binding module 49. For functional characterization, these four cellulases were expressed in Pichia pastoris. All recombinant enzymes demonstrated glucanase activity toward cellulose and hemicellulose substrates. Particularly, VmGH9C1 demonstrated high activity and ability to degrade cellulose, xyloglucan, and glucomannan. In addition, all the enzymes retained activity under wide pH (6-10) and temperature ranges (optimum 70°C), revealing the potential applications of plant GH9 cellulases in the industrial bioprocessing of lignocellulose.
Hide AbstractCoconut rhinoceros beetle digestive symbiosis with potential plant cell wall degrading microbes.
Han, C. J., Cheng, C. H., Yeh, T. F., Pauchet, Y. & Shelomi, M. (2024). npj Biofilms and Microbiomes, 10(1), 34.
Coconut rhinoceros beetle (CRB, Oryctes rhinoceros) is an invasive palm pest whose larvae eat wood, yet lack the necessary digestive enzymes. This study confirmed endogenous CRB cellulase is inactive, suggesting microbial fermentation. The inner lining of the CRB hindgut has tree-like structures covered with a conspicuous biofilm. To identify possible symbionts, 16 S rRNA amplicon sequencing was used on individuals from across Taiwan. Several taxa of Clostridia, an anaerobic class including many cellulolytic bacteria, were highly abundant in most individuals from all locations. Whole metagenome sequencing further confirmed many lignocellulose degrading enzymes are derived from these taxa. Analyses of eggs, larvae, adults, and soil found these cellulolytic microbes are not transmitted vertically or transstadially. The core microbiomes of the larval CRB are likely acquired and enriched from the environment with each molt, and enable efficient digestion of wood.
Hide AbstractParasitic dodder expresses an arsenal of secreted cellulases with multi-substrate specificity during host invasion.
Edema, H., Bawin, T., Olsen, S., Krause, K. & Karppinen, K. (2024). Plant Physiology and Biochemistry, 210, 108633.
Cuscuta campestris is a common and problematic parasitic plant which relies on haustoria to connect to and siphon nutrients from host plants. Glycoside hydrolase family 9 (GH9) cellulases (EC 3.2.1.4) play critical roles in plant cell wall biosynthesis and disassembly, but their roles during Cuscuta host invasion remains underexplored. In this study, we identified 22 full-length GH9 cellulase genes in C. campestris genome, which encoded fifteen secreted and seven membrane-anchored cellulases that showed distinct phylogenetic relationships. Expression profiles suggested that some of the genes are involved in biosynthesis and remodeling of the parasite's cell wall during haustoriogenesis, while other genes encoding secreted B- and C-type cellulases are tentatively associated with degrading host cell walls during invasion. Transcriptomic data in a host-free system and in the presence of susceptible or partially resistant tomato hosts, showed for especially GH9B7, GH9B11 and GH9B12 a shift in expression profiles in the presence of hosts, being more highly expressed during host attachment, indicating that Cuscuta can tune cellulase expression in response to a host. Functional analyses of recombinant B- and C-type cellulases showed endoglucanase activities over wide pH and temperature conditions, and activities towards multiple cellulose and hemicellulose substrates. These findings improve our understanding of host cell wall disassembly by Cuscuta, and cellulase activity towards broad substrate range potentially explain its wide host range. This is the first study to provide a broad biochemical insight into Cuscuta GH9 cellulases, which based on our study may have potential applications in industrial bioprocessing.
Hide AbstractFunctional characterisation of a new halotolerant seawater active glycoside hydrolase family 6 cellobiohydrolase from a salt marsh.
Leadbeater, D. R. & Bruce, N. C. (2024). Scientific Reports, 14(1), 3205.
Realising a fully circular bioeconomy requires the valorisation of lignocellulosic biomass. Cellulose is the most attractive component of lignocellulose but depolymerisation is inefficient, expensive and resource intensive requiring substantial volumes of potable water. Seawater is an attractive prospective replacement, however seawater tolerant enzymes are required for the development of seawater-based biorefineries. Here, we report a halophilic cellobiohydrolase SMECel6A, identified and isolated from a salt marsh meta-exo-proteome dataset with high sequence divergence to previously characterised cellobiohydrolases. SMECel6A contains a glycoside hydrolase family 6 (GH6) domain and a carbohydrate binding module family 2 (CBM2) domain. Characterisation of recombinant SMECel6A revealed SMECel6A to be active upon crystalline and amorphous cellulose. Mono- and oligosaccharide product profiles revealed cellobiose as the major hydrolysis product confirming SMECel6A as a cellobiohydrolase. We show SMECel6A to be halophilic with optimal activity achieved in 0.5X seawater displaying 80.6 ± 6.93% activity in 1 × seawater. Structural predictions revealed similarity to a characterised halophilic cellobiohydrolase despite sharing only 57% sequence identity. Sequential thermocycling revealed SMECel6A had the ability to partially reversibly denature exclusively in seawater retaining significant activity. Our study confirms that salt marsh ecosystems harbour enzymes with attractive traits with biotechnological potential for implementation in ionic solution based bioprocessing systems.
Hide AbstractCoarse-grained molecular dynamics model to evaluate the mechanical properties of bacterial cellulose–hemicellulose composites.
Chibrikov, V., Pieczywek, P. M., Cybulska, J. & Zdunek, A. (2024). Carbohydrate Polymers, 330, 121827.
The plant cell wall (PCW) inspires the preparation of fiber-based biomaterials, particularly emphasizing exploiting the intrinsic interactions within the load-bearing cellulose and hemicellulose network. Due to experimental difficulties in studying and interpreting the interaction between these polysaccharides, this research presents a numerical model based on coarse-grained molecular dynamics that evaluates the mechanical properties of fiber composites. To validate the model and explain the structural and mechanical role of hemicelluloses, bacterial cellulose (BC) was synthesized in the presence of different concentrations of xylan, arabinoxylan, xyloglucan, or glucomannan and subjected to nano- and macroscale structural and mechanical characterization. The data obtained were used to interpret the effects of each hemicellulose on the mechanics of the BC–hemicellulose composite based on the sensitivity of the model. The mechanical properties of the resulting simulated networks agreed well with the experimental observations of the BC–hemicellulose composites. Increased xylan and arabinoxylan contents increased the macroscale mechanical properties, fiber modulus (xylan), and fiber width (arabinoxylan). The addition of xyloglucan increased the mechanical properties of the composites in the elastic deformation phase, associated with an increase in the fiber modulus. Adding glucomannan to the culture medium decreased all the mechanical properties studied while the fiber width increased.
Hide Abstract