| Content: | 200 assays per kit (4 vials) |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | β-Amylase |
| Assay Format: | Spectrophotometer |
| Detection Method: | Absorbance |
| Wavelength (nm): | 400 |
| Limit of Detection: | 0.05 U/mL |
| Reproducibility (%): | ~ 3% |
| Reaction Time (min): | ~ 10 min |
High purity Betamyl-3; β-Amylase Assay Reagent – 4 vials for the measurement of β-amylase for research, biochemical enzyme assays and analytical testing applications.
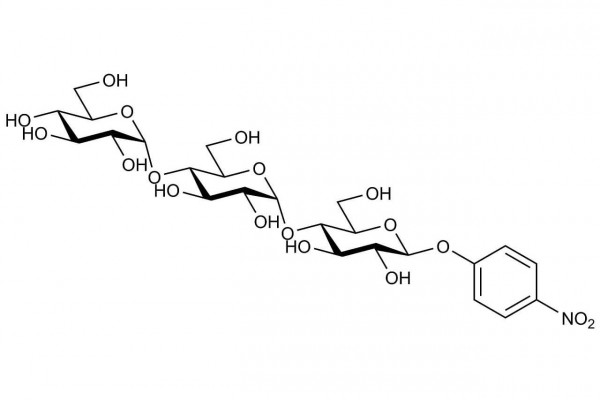
p-Nitrophenyl β-D-maltotrioside, plus excess thermostable β-glucosidase (stabilised reagent).
We offer a wide range of reagent mixture and assay kits.
McCleary, B. V. & Codd, R. (1989). Journal of Cereal Science, 9(1), 17-33.
A procedure previously developed for the assay of cereal-flour β-amylase has been improved and standardised. The improved procedure uses the substrate p-nitrophenyl maltopentaose (PNPG5) in the presence of near saturating levels of α-glucosidase. PNPG5 is rapidly hydrolysed by β-amylase but less readily by cereal α-amylases. The substrate is hydrolysed by β-amylase to maltose and p-nitrophenyl maltotriose (PNPG3). With the levels of α-glucosidase used in the substrate mixture, PNPG3 is rapidly cleaved to glucose and p-nitrophenol, whereas PNPG5 is resistant to hydrolysis by the α-glucosidase. The assay procedure has been standardised for several β-amylases and the exact degree of interference by cereal α-amylases determined. The procedure can be readily applied to the selective measurement of β-amylase activity in cereal and malted cereal-flours.
Hide AbstractAn Extremely Sensitive Amylase Activity Assay and its Application for the Determination of the Residual Amylase Activity in Bread.
Reichenberger, K., Lutz-Wahl, S., Kettner, L. & Fischer, L. (2025). Food Analytical Methods, 1-13.
An extremely sensitive amylase activity assay was developed using the natural substrate starch and two ancillary enzymes: a glucose oxidase (GOD) and a peroxidase, to measure the residual activity of the α-amylase from Bacillus subtilis in white bread. Firstly, the concentrations of the assay components: electron acceptor DA-67 (50 μM), horseradish peroxidase (681 nkat mL−1), a GOD from Aspergillus niger (1550 nkat mL−1) and the natural substrate starch (0.01% (w/v)), were optimized to achieve high sensitivity. The linearity of the assay was then tested with both an endo- (α-amylase from B. subtilis) and exo-acting amylase (maltogenic amylase from Geobacillus stearothermophilus), and the effect of the incubation time on the assay sensitivity was investigated and optimized. The optimized assay was capable of determining a minimal amylase activity of 0.33 pkat mL−1 for both amylases tested with an assay run time of 7.5 h. This new DA-67 amylase assay demonstrated 4.7- and 4.2-times higher sensitivity, respectively, compared to optimized versions of the commercial Ceralpha (determination of endo-amylase activities) and Betamyl3 (determination of exo-amylase activities) assays. The new DA-67 amylase assay was used to determine the residual activity of α-amylase from B. subtilis in white bread. A consistent residual activity of 2.26 ± 0.15% was reliably determined.
Hide AbstractImpact of exogenous α-amylases on sugar formation in straight dough wheat bread.
Rebholz, G. F., Sebald, K., Dirndorfer, S., Dawid, C., Hofmann, T. & Scherf, K. A. (2020). European Food Research and Technology, 1-12.
The use of bacterial or fungal α-amylases is common in wheat bread production to improve several quality-related parameters such as loaf volume, crust color or staling behavior. To study the impact of exogenous α-amylases on straight dough wheat bread, we quantitated mono-, di- and oligosaccharides and residual α-amylase activity in bread crumb during storage for up to 96 h. Discovery-driven proteomics of the five α-amylase preparations studied showed that only a few different amylases per preparation were responsible for the hydrolytic effect. Compared to the control, the supplementation with α-amylase from Bacillus amyloliquefaciens in wheat dough preparation led to major changes in the sugar composition of bread crumb during storage with the formation of oligosaccharides like maltopentaose, maltohexaose, maltoheptaose, and maltooctaose. A residual activity corresponding to 4.0% of the applied activity was determined in the breads prepared with α-amylase from B. amyloliquefaciens, but no residual activity was detected for any of the other fungal or bacterial α-amylases from Aspergillus oryzae or Thermoactinomyces vulgaris. Whether the detected residual activity is related to the characteristics of bread staling or bread crumb properties must be clarified in further studies.
Hide AbstractSchnitzenbaumer, B. & Arendt, E. K. (2014). European Food Research and Technology, 238(2), 225-235.
Barley malt is the preferred brewing material these days because of its high extract content and high enzyme activities. However, when substituting malted barley with oats to create a unique beer flavor and aroma, endogenous malt enzymes become the limiting factor. Therefore, the objectives of this study were to evaluate the effect of 10–40% unmalted oats on the quality of high-gravity mashes/worts and to investigate the limitations of endogenous malt enzymes as well as the benefits of the application of industrial enzymes. The enzyme mix Ondea® Pro was found to be particularly suitable for mashing with unmalted oats and was therefore used in the present rheological tests and laboratory-scale mashing trials. In order to gain detailed information about the biochemical processes occurring during mashing, the quality of mashes was comprehensively analyzed after each mash rest using standard methods described by Mitteleuropäische Brautechnische Analysenkommission and Lab-on-a-Chip capillary electrophoresis. Mashing with up to 40% oats resulted in increased mash consistencies, color/pH (20°C) values, β-glucan concentrations, wort viscosities 12.0%, and filtration times as well as decreased FAN and extract contents. The application of Ondea® Pro enormously increased the color of worts despite lower pH values but considerably improved the quality and processability of 30 or 40% oat-containing mashes/worts. However, the substitution of up to 20% barley malt with unmalted oats can easily be realized without the addition of exogenous enzymes.
Hide AbstractGermination of oat and quinoa and evaluation of the malts as gluten free baking ingredients.
Mäkinen, O. E., Zannini, E. & Arendt, E. K. (2013). Plant Foods for Human Nutrition, 68(1), 90-95.
Germination can be used to improve the sensory and nutritional properties of cereal and pseudocereal grains. Oat and quinoa are rich in minerals, vitamins and fibre while quinoa also contains high amounts of protein of a high nutritional value. In this study, oat and quinoa malts were produced and incorporated in a rice and potato based gluten free formulation. Germination of oat led to a drastic increase of α-amylase activity from 0.3 to 48 U/g, and minor increases in proteolytic and lipolytic activities. Little change was observed in quinoa except a decrease in proteolytic activity from 9.6 to 6.9 U/g. Oat malt addition decreased batter viscosities at both proofing temperature and during heating. These changes led to a decrease in bread density from 0.59 to 0.5 g/ml and the formation of a more open crumb, but overdosing of oat malt deteriorated the product as a result of excessive amylolysis during baking. Quinoa malt had no significant effect on the baking properties due to low α-amylase activity. Despite showing a very different impact on the bread quality, both malts influenced the electrophoretic patterns of rice flour protein similarly. This suggests that malt induced proteolysis does not influence the technological properties of a complex gluten free formulation.
Hide AbstractOliveira, P. M., Mauch, A., Jacob, F., Waters, D. M. & Arendt, E. K. (2012). International Journal of Food Microbiology, 156(1), 32-43.
Barley infection with Fusarium species has been a long standing problem for the malting and brewing industries. In this study, we evaluate the impact of Fusarium culmorum infected raw barley on the final malt quality. Barley grains were infected for 5 days at optimum fungal growth conditions. Grains were fully characterized and compared to standard barley grains. Due to fungal infection, germinative energy of infected barley grains decreased by 45%; its water sensitivity increased dramatically, and grains accumulated 199 µg/kg of deoxynivalenol (DON). Barley grains were subsequently malted for 8 days, fully characterized and compared to standard malt grains. Fungal growth behavior was evaluated during malting using a PCR-based assay and mycotoxins were measured using HPLC. Fungal biomass increased in grains, during all stages of malting. Infected malt accumulated 8-times its DON concentration during malting. Kernel ultrastructure was evaluated using scanning electron and confocal laser scanning microscopy. Infected malt grains were characterized by extreme structural proteolytic, (hemi)-cellulolytic and starch deterioration with increased friability and fragmentation. Infected grains had higher protease and β-glucanase activities, lower amylase activity, a greater proportion of free amino and soluble nitrogen, and a lower β-glucan content. Malt loss was over 27% higher in infected malt in comparison to the control. The results of this study revealed that 20% F. culmorum infected barley kernels lead to a significant reduction in malt quality as well as mycotoxin formation.
Hide AbstractEvans, D. E., Dambergs, R., Ratkowsky, D., Li, C., Harasymow, S., Roumeliotis, S. & Eglinton, J. K. (2010). Journal of the Institute of Brewing, 116(1), 86-96.
Prediction of malt fermentability (apparent attenuation limit — AAL) by measurement of the diastatic power enzymes (DPE), α-amylase, total limit dextrinase, total β-amylase, β-amylase thermostability, and the Kolbach index (KI or free amino nitrogen — FAN) is superior to the conventional use of diastatic power (DP) alone. The thermostability of β-amylase is known to be an important factor in determining fermentability, thus the thermostability of the other relatively thermolabile enzyme, limit dextrinase, was investigated to determine if it was also useful in predicting fermentability. To facilitate this aim, methods were developed for a rapid and cost efficient assay of both β-amylase and limit dextrinase thermostability. Internationally important Australian and international malting varieties were compared for their total limit dextrinase and β-amylase activity and thermostability. Interestingly, the level of limit dextrinase thermostability was observed to be inversely correlated with total limit dextrinase activity. The prediction of malt fermentability was achieved by both forward step-wise multi-linear regression (MLR) and the partial least squares (PLS) multivariate model development methods. Both methods produced similar identifications of the parameters predicting wort fermentability at similar levels of predictive power. Both models were substantially better at predicting fermentability than the traditional use of DP on its own. The emphasis of this study was on the identification of predictive factors that can be consistently used in models to predict fermentability, because the model parameter estimates will subtly vary depending on mashing conditions, yeast strain/fermentation conditions and malt source. The application of these multivariate model development methods (PLS and MLR) enabled the identification of further potential fermentability predicting factors. The analyses divided the predictive parameters into those defined by DP enzymes and those associated with modification (KI, FAN, fine/coarse difference, wort β-glucan and friability). Surprisingly, limit dextrinase thermostability was not a substantial predictor of fermentability, presumably due to its negative correlation with total limit dextrinase activity. The application of these insights in the malting and brewing industries is expected to result in substantial improvements in brewing consistency and enable more specific quality targets for barley breeder's progeny selection cut-off limits to be more precisely defined.
Hide Abstract