| Content: | 5 g |
| Shipping Temperature: | Ambient |
| Storage Temperature: | Ambient |
| Physical Form: | Powder |
| Stability: | > 2 years under recommended storage conditions |
| CAS Number: | 9041-22-9 |
| Source: | Barley flour |
| Molecular Weight: | 495,000 |
| Purity: | > 90% |
| Viscosity: | High > 100 cSt |
| Monosaccharides (%): | Glucose = 94 |
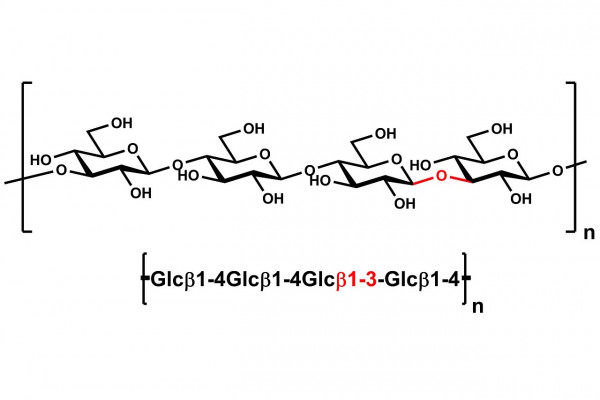
| Main Chain Glycosidic Linkage: | β-1,4 and β-1,3 |
| Substrate For (Enzyme): | β-Glucanase/Lichenase |
High purity β-Glucan (Barley; High Viscosity) for use in research, biochemical enzyme assays and analytical testing applications.
High viscosity β-Glucan from barley flour.
Looking for other products? See our list of polysaccharide products.
Hughes, S. A., Shewry, P. R., Gibson, G. R., McCleary, B. V. & Rastall, R. A. (2008). FEMS Microbiology Ecology, 64(3), 482-493.
Fermentation of β-glucan fractions from barley [average molecular mass (MM), of 243, 172, and 137 kDa] and oats (average MM of 230 and 150 kDa) by the human faecal microbiota was investigated. Fractions were supplemented to pH-controlled anaerobic batch culture fermenters inoculated with human faecal samples from three donors, in triplicate, for each substrate. Microbiota changes were monitored by fluorescent in situ hybridization; groups enumerated were: Bifidobacterium genus, Bacteroides and Prevotella group, Clostridium histolyticum subgroup, Ruminococcus-Eubacterium-Clostridium (REC) cluster, Lactobacillus-Enterococcus group, Atopobium cluster, and clostridial cluster IX. Short-chain fatty acids and lactic acid were measured by HPLC. The C. histolyticum subgroup increased significantly in all vessels and clostridial cluster IX maintained high populations with all fractions. The Bacteroides-Prevotella group increased with all but the 243-kDa barley and 230-kDa oat substrates. In general β-glucans displayed no apparent prebiotic potential. The SCFA profile (51 : 32 : 17; acetate : propionate : butyrate) was considered propionate-rich. In a further study a β-glucan oligosaccharide fraction was produced with a degree of polymerization of 3-4. This fraction was supplemented to small-scale faecal batch cultures and gave significant increases in the Lactobacillus-Enterococcus group; however, the prebiotic potential of this fraction was marginal compared with that of inulin.
Hide AbstractMcCleary, B. V. & Codd, R. (1991). Journal of the Science of Food and Agriculture, 55(2), 303-312.
A commercially available enzymic method for the quantitative measurement of (1→3),(1→4)-β-glucan has been simplified to allow analysis of up to 10 grain samples in 70 min or of 100–200 samples by a single operator in a day. These improvements have been achieved with no loss in accuracy or precision and with an increase in reliability. The glucose oxidase/peroxidase reagent has been significantly improved to ensure colour stability for periods of up to 1 h after development. Some problems experienced with the original method have been addressed and resolved, and further experiments to demonstrate the quantitative nature of the assay have been designed and performed.
Hide AbstractMcCleary, B. V. & Nurthen, E. (1986). Journal of the Institute of Brewing, 92(2), 168-173.
A method developed for the quantification of (1→3)(1→4)-β-D-glucan in barley flour has been modified to allow its use in the measurement of this component in malt, wort, beer and spent grain. For malt samples, free D-glucose was first removed with aqueous ethanol. Quantification of the polymer in wort and beer samples involved precipitation of the β-glucan with ammonium sulphate followed by washing with aqueous ethanol to remove free D-glucose. Spent grain was lyophilised and milled and then analysed by the method developed for malt. In all cases, the β-glucan was depolymerised with lichenase and the resultant β-gluco-oligosaccharides hydrolysed to D-glucose with β-D-glucosidase. The released D-glucose was then specifically determined using glucose oxidase-peroxidase reagent.
Hide AbstractMcCleary, B. V., Gibson, T. S., Allen, H. & Gams, T. C. (1986). Starch, 38(12), 433-437.
Mixed linkage β-glucane and pentosanes (mainly arabinoxylanes) are the major endosperm cell-wall polysaccharides of barley and wheat respectively. These polysaccharides, although minor components of the whole grain, significantly affect the industrial utilization of these cereals. The modification of barley corns during malting requires the dissolution of the β-glucan in the cell-wall of the starch endosperm. High β-glucane concentration in wort and beer effect the rate of filtration and can also lead to precipitate or gel formation in the final product. In a similar manner, pentosane is thought to cause filtration problems with wheat starch hydrolysates by increasing viscosity and by producing gelatinous precipitate which blocks filters. Ironically, it is this same viscosity building and water binding capacity which is considered to render pentosanes of considerable value in dough development and bread storage (anti-staling functions). In the current paper, some aspects of the beneficial and detrimental effects of pentosans and β-glucan in the industrial utilization of wheat and barley are discussed. More specifically, enzymic methods for the preparation, analysis and identification of these polysaccharides and for the removal of their functional properties, are described in detail.
Hide AbstractMcCleary, B. V. & Glennie-Holmes, M. (1985). Journal of the Institute of Brewing, 91(5), 285-295.
A simple and quantitative method for the determination of (1→3) (1→4)-β-D-glucan in barley flour and malt is described. The method allows direct analysis of β-glucan in flour and malt slurries. Mixed-linkage β-glucan is specifically depolymerized with a highly purified (1→3) (1→4)-β-D-glucanase (lichenase), from Bacillus subtilis, to tri-, tetra- and higher degree of polymerization (d.p.) oligosaccharides. These oligosaccharides are then specifically and quantitatively hydrolysed to glucose using purified β-D-glucosidase. The glucose is then specifically determined using glucose oxidase/peroxidase reagent. Since barley flours contain only low levels of glucose, and maltosaccharides do not interfere with the assay, removal of low d.p. sugars is not necessary. Blank values are determined for each sample allowing the direct measurement of β-glucan in values are determined for each sample allowing the direct measurement of β-glucan in malt samples. α-Amylase does not interfere with the assay. The method is suitable for the routine analysis of β-glucan in barley samples derived from breeding programs; 50 samples can be analysed by a single operator in a day. Evaluation of the technique on different days has indicated a mean standard error of 0-1 for barley flour samples containing 3-8 and 4-6% (w/w) β-glucan content.
Hide AbstractImpact of Non-Starch Polysaccharides and Protein on Mash Viscosity: A Comparative Study of Hard and Soft Wheat in Irish Whiskey Production.
Vashishtha, A., Ryan, L., Whelan, S., Byrne, J., Garcia-Cabellos, G. & Morris, S. (2025). Journal of the American Society of Brewing Chemists, 1-9.
The viscosity of wheat mash is a critical parameter in Irish whiskey production, directly influencing mashing efficiency and lautering. Elevated viscosity can result in operational issues such as mash thickening, reduced pumpability, and equipment fouling. This study investigates the role of Non-Starch Polysaccharides (NSP)-specifically Water Extractable Arabinoxylans (WEAX) and β-glucans-on the viscosity of mash produced from hard and soft wheat at various mashing stages. Additionally, the relationship between protein content and viscosity is analysed to understand its role in mash processing challenges. A time-point study was conducted to measure viscosity, NSP levels, and protein content at different stages of mashing for both hard and soft wheat. Statistical analysis using the Generalised Linear Model (GLM) demonstrated a significant positive relationship between β-glucan and viscosity in soft wheat, whereas WEAX had an inverse relationship with viscosity for soft wheat. NSP did not show any statistically significant effect on the viscosity of hard wheat. Protein content, on the other hand, had a highly significant inverse relationship with viscosity in both hard and soft wheat. Despite lower β-glucan and WEAX extraction levels in hard wheat, its viscosity remained consistently higher than that of soft wheat, suggesting that the molecular weight of the extracted NSP, rather than their concentration alone, may play a critical role in influencing mash rheology. The findings of this study are important in balancing the NSP and protein levels to control viscosity in wheat-based whiskey production, providing key insights to enhance grain-based whiskey production.
Hide AbstractStrong Association between Proanthocyanidins and Polysaccharides in the Cell Walls of Western Redcedar Bark.
Bautista, G. F. M., Musl, O., Easson, M. L., Kruse, L. H., Gordon, H., Bacher, M., Sumerskii, I., Watrelot, A. A., Bohlmann, J., Potthast, A., Rosenau, T. & Rojas, O. J. (2025). Biomacromolecules.
The co-occurrence of polysaccharides and proanthocyanidins in the aqueous extracts of western redcedar (Thuja plicata Donn; WRC) bark limits their commercial utilization. To better understand their association, proanthocyanidins and polysaccharides were extracted with cold water (3.4% w/w bark) and isolated as an alcohol-insoluble residue (AIR, 1.0% w/w bark). The polysaccharide content (~30% w/w AIR) was analyzed by acidic and enzymatic depolymerization, revealing the presence of pectins, xyloglucans, and xylans. NMR spectroscopy identified features, such as acetylation and methyl esterification. Thiolysis followed by HPLC-DAD revealed that proanthocyanidins (1.46% w/w AIR) exhibit a mean degree of polymerization of 5.3, a cis/trans ratio of 0.40, and a procyanidin/prodelphinidin ratio of 3.90. This study provides a detailed structural characterization of proanthocyanidins and polysaccharides in the AIR of WRC bark. The findings highlight their strong association, which may contribute to distinctive properties that warrant further exploration, particularly in efforts to valorize bark residues.
Hide AbstractImpact of condensed tannin interactions with grain proteins and non-starch polysaccharides on batter system properties.
Girard, A. L. & Awika, J. M. (2021). Food Chemistry, 359, 129969.
Proanthocyanidins (PA) cross-link wheat gluten proteins and dramatically enhance batter viscosity; PA could similarly affect related grains. This study aimed to determine PA effect on viscosity and pasting properties of barley, rye, and oat flours, and the relative contributions of PA interactions with proteins and non-starch polysaccharides (NSP). PA significantly increased batter viscosity, stability, and RVA peak viscosity in rye and barley flours (2.8× and 1.2×, respectively). Interestingly, viscosity peaked distinctively ~75°C in PA-treated rye and barley flours, and their isolated protein-starch systems, indicating prolamins unravelled and complexed with PA during heating. Oat was largely unaffected by PA, likely because of its protein composition. Furthermore, water-soluble rye NSP and arabinoxylans, but not barley β-glucans, significantly increased starch pasting viscosity with PA; oxidative gelation was not a factor. Thus, rye flour viscosity dramatically increased through interactive effects of PA on rye proteins and NSP, which could expand its food applications.
Hide AbstractXyloglucan Is Not Essential for the Formation and Integrity of the Cellulose Network in the Primary Cell Wall Regenerated from Arabidopsis Protoplasts.
Kuki, H., Yokoyama, R., Kuroha, T. & Nishitani, K. (2020). Plants, 9(5), 629.
The notion that xyloglucans (XG) play a pivotal role in tethering cellulose microfibrils in the primary cell wall of plants can be traced back to the first molecular model of the cell wall proposed in 1973, which was reinforced in the 1990s by the identification of Xyloglucan Endotransglucosylase/Hydrolase (XTH) enzymes that cleave and reconnect xyloglucan crosslinks in the cell wall. However, this tethered network model has been seriously challenged since 2008 by the identification of the Arabidopsis thaliana xyloglucan-deficient mutant (xxt1 xxt2), which exhibits functional cell walls. Thus, the molecular mechanism underlying the physical integration of cellulose microfibrils into the cell wall remains controversial. To resolve this dilemma, we investigated the cell wall regeneration process using mesophyll protoplasts derived from xxt1 xxt2 mutant leaves. Imaging analysis revealed only a slight difference in the structure of cellulose microfibril network between xxt1 xxt2 and wild-type (WT) protoplasts. Additionally, exogenous xyloglucan application did not alter the cellulose deposition patterns or mechanical stability of xxt1 xxt2 mutant protoplasts. These results indicate that xyloglucan is not essential for the initial assembly of the cellulose network, and the cellulose network formed in the absence of xyloglucan provides sufficient tensile strength to the primary cell wall regenerated from protoplasts.
Hide AbstractComparative prebiotic activity of mixtures of cereal grain polysaccharides.
Harris, S., Monteagudo-Mera, A., Kosik, O., Charalampopoulos, D., Shewry, P. & Lovegrove, A. (2019). AMB Express, 9(1), 203.
The main components of the non-starch polysaccharide (NSP) fraction of wheat flour are arabinoxylan (AX) and β-glucan. These are also present in other cereal grains, but their proportions vary with AX being the major component in wheat and rye and β-glucan in barley and oats. Therefore, it was hypothesised that these NSPs could act synergistically when fermented in vitro at the ratios present in the major foods consumed, resulting in increased prebiotic activity. AX and β-glucan were therefore tested in in vitro fermentation studies to assess their prebiotic activity when used individually and/or in different ratios. Short-chain fatty-acids (SCFAs) produced from in vitro fermentation were measured using HPLC and bacterial populations were measured using flow cytometry with fluorescence in situ hybridisation (Flow-FISH). Fermentation of AX alone resulted in a significant bifidogenic activity and increased concentrations of SCFAs, mainly acetate, after 8-24 h of fermentation, however β-glucan alone did not show prebiotic activity. The greatest prebiotic activity, based on concentration of total SCFAs and increases in total bacteria as well as beneficial Bifidobacterium and Clostridium coccoides /Eubacterium groups, was observed when AX and β-glucan were combined at a 3:1 ratio, which corresponds to their ratios in wheat flour which is major source of cereal fibre in the diet. This indicates that the population of bacteria in the human GI tract may be modulated by the composition of the fibre in the diet, to maximise the prebiotic potential.
Hide Abstract