| Content: | 5,000 Units |
| Shipping Temperature: | Ambient |
| Storage Temperature: | 2-8oC |
| Formulation: | In 3.2 M ammonium sulphate |
| Physical Form: | Suspension |
| Stability: | > 4 years at 4oC |
| Enzyme Activity: | Other Activities |
| EC Number: | 2.6.1.1 |
| CAS Number: | 9000-97-9 |
| Synonyms: | aspartate transaminase; L-aspartate:2-oxoglutarate aminotransferase |
| Source: | Escherichia coli |
| Molecular Weight: | 45,737 |
| Concentration: | Supplied at ~ 2,500 U/mL |
| Expression: | Recombinant from Escherichia coli |
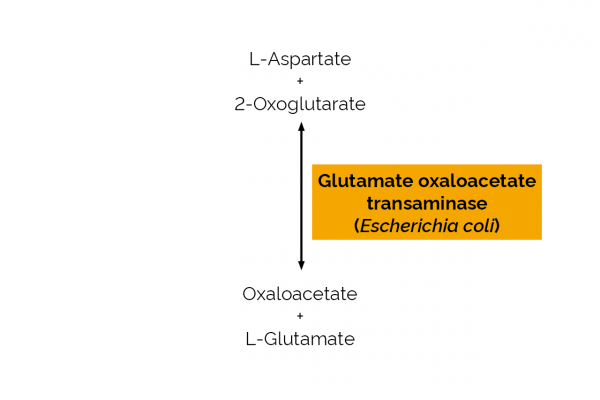
| Specificity: | Catalyses the reversible transfer of an α-amino group between aspartate and glutamate. |
| Specific Activity: | ~ 200 U/mg (25oC, pH 8.5 on α-ketoglutaric acid) |
| Unit Definition: | One Unit of L-glutamic-oxaloacetic transaminase activity is defined as the amount of enzyme required to convert one µmole of α-ketoglutarate to L-glutamate per minute in the presence of NADH at pH 8.5 and 25oC. |
| Temperature Optima: | 25oC |
| pH Optima: | 8.5 |
| Application examples: | Applications in diagnostics and analytical methods in the food and feeds, fermentation, beverages and wine industries. |
This product has been discontinued (read more).
High purity Glutamate oxaloacetate transaminase (Escherichia coli) for use in research, biochemical enzyme assays and in vitro diagnostic analysis.
See more of our high purity analytical enzymes.
Zhang, D., Qi, Y., Klyubin, I., Ondrejcak, T., Sarell, C. J., Cuello, A. C., Collinge, J. & Rowan, M. J. (2017). Neuropharmacology, 121, 231-246.
Alzheimer's disease amyloid-β (Aβ) oligomers are synaptotoxic, inappropriately increasing extracellular glutamate concentration and glutamate receptor activation to thereby rapidly disrupt synaptic plasticity. Thus, acutely promoting brain glutamate homeostasis with a blood-based scavenging system, glutamate-oxaloacetate transaminase (GOT), and blocking metabotropic glutamate 5 (mGlu5) receptor or its co-receptor cellular prion protein (PrP), prevent the acute inhibition of long-term potentiation (LTP) by exogenous Aβ. Here, we evaluated the time course of the effects of such interventions in the persistent disruptive effects of Aβ oligomers, either exogenously injected in wild type rats or endogenously generated in transgenic rats that model Alzheimer's disease amyloidosis. We report that repeated, but not acute, systemic administration of recombinant GOT type 1, with or without the glutamate co-substrate oxaloacetate, reversed the persistent deleterious effect of exogenous Aβ on synaptic plasticity. Moreover, similar repetitive treatment reversibly abrogated the inhibition of LTP monitored longitudinally in freely behaving transgenic rats. Remarkably, brief repeated treatment with an mGlu5 receptor antagonist, basimglurant, or an antibody that prevents Aβ oligomer binding to PrP, ICSM35, also had similar reversible ameliorative effects in the transgenic rat model. Overall, the present findings support the ongoing development of therapeutics for early Alzheimer's disease based on these complementary approaches.
Hide Abstract