200 assays (manual) / 2000 assays (microplate) / 1960 assays (auto-analyser)
| Content: | 200 assays (manual) / 2000 assays (microplate) / 1960 assays (auto-analyser) |
| Shipping Temperature: | Ambient |
| Storage Temperature: |
Short term stability: 2-8oC, Long term stability: See individual component labels |
| Stability: | > 2 years under recommended storage conditions |
| Analyte: | Glucose Oxidase |
| Assay Format: | Spectrophotometer, Microplate, Auto-analyser |
| Detection Method: | Absorbance |
| Wavelength (nm): | 510 |
| Signal Response: | Increase |
| Linear Range: | 0.01 to 0.08 U/mL of glucose oxidase per assay |
| Limit of Detection: | 10 U/L |
| Reaction Time (min): | ~ 20 min |
| Application examples: | Enzyme preparations, and other materials (e.g. biological cultures, samples, etc.). |
| Method recognition: | Novel method |
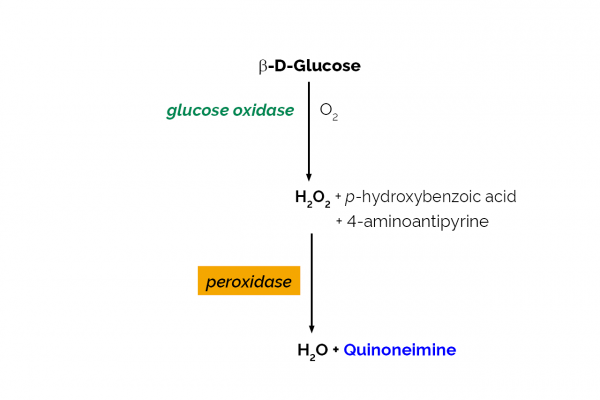
The Glucose Oxidase assay kit is a simple procedure for the rapid and reliable measurement and analysis of glucose oxidase activity in industrial enzyme preparations and bread improver mixtures.
View more of our assay kits for enzyme activities.

- Very competitive price (cost per test)
- All reagents stable for > 12 months after preparation
- Simple format
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
- Suitable for manual, microplate and auto-analyser formats
The supplementation of dietary black cumin (Nigella Sativa) seeds on performance, blood hematology, post-metabolic responses, antioxidant status, immunity, and inflammatory markers in pre-weaning calves.
Elsayed, M., Al-Marakby, K. M., Abdel Hafez, S. & Abdelnour, S. A. (2025). Tropical Animal Health and Production, 57(3), 1-11.
Pre-weaning feeding is critical for calf growth, laying the foundation for future productivity and health. Nigella sativa seeds (NS) are rich in bioactive compounds with numerous beneficial effects on health and various pharmacological properties. This study aimed to investigate the supplementation of NS powder on performance, post-metabolic attributes, immunity, antioxidant capacity, and inflammatory responses in pre-weaning Friesian calves. Twenty-four Friesian male calves at 4 days of age with a similar genetic line, weighing 33.67 ± 0.6 kg, were randomly allocated to three groups (8 animals per group). The study comprised three groups: a control group (NS0) receiving no supplementation, and two experimental groups received either 1% (NS1) or 3% (NS3) NS supplementation for 84 days. All levels of NS supplementation significantly improved the final body weight and body weight gain in a linear manner (P < 0.001), while the highest total dry matter intake was observed in NS1 group (quadratic; P < 0.001). White blood cells (quadratic, P = 0.026), lymphocytes (quadratic, P = 0.012), and monocytes (linear effect; P = 0.001) significantly decreased, whereas red blood cells (linear; P = 0.004), hematocrit (linear; P = 0.002), mean corpuscular volume (MCV, linear; P = 0.003), and mean corpuscular hemoglobin concentration (MCHC, quadratic, P = 0.007), platelets (linear; P < 0.001) increased in calves fed NS. Feeding calves diets supplemented with NS led to a significant linear decrease in plasma creatinine and liver enzymes (AST and ALT) compared to the control diet (P < 0.01). Calves fed 3% of NS in their diets had lower plasma cholesterol (linear; P < 0.001) and triglyceride levels (linear; P = 0.002) compared to calves in NS0 and NS1 groups. Polynomial analysis indicated a quadratic decrease in direct bilirubin (P = 0.006), and a quadratic increase in immunoglobulin G (IgG, P = 0.014) and M (IgM, P = 0.032) in the calves fed the NS diet. All NS-supplemented groups showed a significant increase in IL-10 (linear; P < 0.001), TAC (linear; P = 0.006), and CAT (linear; P < 0.001), and a significant decrease in IL-4 levels (linear; P < 0.001) of the plasma of pre-weaning calves. As expected, pre-weaning calves fed diets containing NS (1% or 3%) exhibited a quadratic decrease in plasma malondialdehyde (MDA, P < 0.001) levels compared to those fed diets without NS. Our findings suggest that incorporating up to 3% Nigella sativa into pre-weaning calf diets can enhance growth, bolster immune function, and mitigate oxidative stress, offering a promising strategy for improving health and sustainability on dairy farms.
Hide AbstractThe comparison of the antioxidant, antibacterial and antiviral potential of Polish fir honeydew and Manuka honeys.
Grabek-Lejko, D., Miłek, M. & Dżugan, M. (2024). Scientific Reports, 14(1), 31170.
The aim of the present study was to compare the antioxidant, antibacterial and antiviral activities of Podkarpackie coniferous honeydew honey and Manuka honey. The quality of tested honey samples (honeydew-12 and Manuka-4) regarding honey standard was evaluated as well as additional indicators (methylglyoxal, total phenolics and HPTLC phenolic profile, antioxidant potential, glucose oxidase activity, and hydrogen peroxide) were compared. Antibacterial potential was analyzed against Gram-positive (S. aureus and B. cereus) and Gram-negative (E. coli and S. enterica) bacteria. Antiviral activity against different RNA (phi6, MS2) and DNA (T7, phiX174) bacteriophages considered as "viral surrogates" was determined. Based on the determined physicochemical parameters the good quality of tested honeys was confirmed, excluding two samples. The content of polyphenolic compounds in honeydew honey ranged from 583.87 to 1102.42 mg of gallic acid/kg and was strongly correlated with the antioxidant properties. Moreover, for samples with the strongest activity these parameters were comparable to Manuka honey. However, the obtained HPTLC polyphenolic profiles were completely different for honeydew than for Manuka honey which exhibited additional bands (Rf = 0.74 and 0.52). Honeydew honeys were characterized by a strong antiviral and antibacterial properties most of all against Gram-positive bacteria. The MICs (minimal inhibitory concentrations) for S. aureus and B. cereus ranged 15-35% and 8-15% for honeydew and Manuka honeys, respectively. The strongest antiviral properties of honeydew honey were demonstrated mainly against RNA bacteriophages (phi6, MS2) which was even higher than for Manuka honey, especially against MS2 virus. The obtained results suggest that Podkarpackie honeydew honey with the controlled glucose oxidase activity may be a natural substance used to combat viral and bacterial diseases.
Hide AbstractFTIR characteristics of sweet flour as affected by particle size distribution and in vitro digestibility of gluten-free sweet potato noodles.
Azeem, M., Wadood, S. A., Qureshi, T. M., Anees Ur Rehman, M., Mohamed Ahmed, I. A., Aljobair, M. O. & Ali, A. A. (2025). International Journal of Food Properties, 28(1), 16-31.
There is a need to explore the influence of particle size of sweet potato flour on physicochemical properties and in vitro starch digestibility of gluten free sweet potato noodles. The aim of this work was to examine the influence of purple-fleshed sweet potato flour (PFSPF) having different particle size distribution (355 µm to 75 µm) on physicochemical properties and in vitro starch digestibility of gluten-free purple sweet potato noodles. Pasting properties and sugar contents were positively correlated toward the finest particle fraction. The hardness of noodles was positively and significantly correlated to the finest particle size fraction. Sweet potato flour (SPF) noodles prepared with particle size of 75 µm retained lower hardness 5.74 N. An abrupt increase in yellowness was observed with decreasing particle size that might be attributed to the reduction of protein. The results of FTIR suggested that with the reduction of particle size, protein and carbohydrates decreased that much associated with crystallization and retrogradation of starch. Among all the amino acids, aspartic acid and glutamic acid were observed to be more abundant, i.e. approximately 13.33 and 29.05%, respectively. In this way, PFSPF with particle size 75 µm exhibited significant application potential for producing gluten free noodles due to high resistant starch and lower hardness. It may be concluded that particle sizes of sweet potato flour influenced pasting properties, textural characteristics, sugar contents, protein contents, amino acid contents, carbohydrate contents of prepared noodles.
Hide AbstractEffects of moisture regulation and heat treatment synergy on structural properties and digestibility of jackfruit seed starch.
Li, S., Dong, S. & Gao, Q. (2024). International Journal of Biological Macromolecules, 282, 137024.
To investigate the impact of moisture regulation and heat treatment synergy on the structural properties and digestibility of jackfruit seed starch (JSS), starch samples underwent heat-moisture treatment (HMT) and annealing treatment (ANN) with varying moisture content (10-30% for HMT at 120°C, and 50-90% for ANN at 40°C). The physicochemical properties and in vitro digestibility of modified-JSS were systematically investigated. Results showed that the birefringence intensity of HMT-JSS decreased at high moisture levels but remained unchanged for HMT-JSS and ANN-JSS at low moisture levels. As moisture content increased for HMT and ANN, the amylose content and relative crystallinity increased and then slightly decreased. The gelatinization temperatures increased while enthalpy and viscosity declined. At high moisture content, the infrared absorbance ratio of 1047 cm−1/1022 cm−1 decreased on HMT but increased on ANN. Resistant starch (RS) contents of both HMT-JSS and ANN-JSS were increased at appropriate moisture levels (10-15% for HMT, 50-80% for ANN), but decreased with excessive moisture. Besides, these changes were more pronounced on HMT than ANN. Correlation analysis showed that the RS was significantly affected by the short-range ordered structure during HMT and ANN. These results revealed that hydrothermal treatment efficiently modified the structure properties and digestibility of JSS.
Hide AbstractAnti-biofilm properties of Portuguese honeys against multi-drug resistant microorganisms: A promising strategy for chronic wounds healing.
Bezerra, A., Alves, M. J., Saavedra, M. J., Russo-Almeida, P., Aires, A., Fonseca, H., Rodrigues, F., Delerue-Matos, C., Garcia, J. & Gouvinhas, I. (2024). Food Bioscience, 61, 104796.
Honey is considered a promising strategy for chronic wound management, being a possible solution for bacterial resistance problem mostly due to its physical-chemical features. However, these properties might vary significantly depending on the honey floral source. Dark-colored honey, such as chestnut honey, is related to increased antioxidant and bactericidal properties. This study aims to assess biofilms of multidrug-resistant microorganisms in honey samples from the Natural Park of Montesinho, in Portugal, which is considered a region with predominant chestnut floral source. The minimum inhibition concentration (MIC) of honey was tested for Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Candida albicans. The biofilm removal as well as the metabolic inactivation of the biofilms were evaluated using three honey concentrations: 1xMIC, 5xMIC, and 10xMIC. The biofilm viability was analyzed with fluorescent microscopy. Honey samples were characterized according to the pollen profile, phenolic composition, H2O2 levels, tyrosinase inhibition, and pH. The antioxidant capacity was determined through FRAP and ABTS assays. Most of the honey samples exhibited a MIC of 6.75%. Biofilm removal efficacy differs among samples. Regression analysis evidenced a positive correlation between the biofilm removal and concentrations of H2O2, vanillic acid, pinocembrin, Cytisus striatus and Corrigiola telephiifolia, and pH. The honey concentration of 10xMIC was the most effective to inhibit the biofilm metabolism and viability. The concentrations of C. sativa, H2O2, and the anti-tyrosinase and FRAP activities were also positively correlated. The honey samples had a dominant presence of Castanea sativa pollen and were effective against biofilms formed by multidrug-resistant microorganisms.
Hide AbstractInhibitory Activity of Red and Yellow Araçá Genotypes Towards Carbohydrate-Hydrolyzing Enzymes: Putative Role of Ellagitannins.
Vinholes, J., Lemos, G., Azambuja, J. H., Reis, S. F., Rudnitskaya, A., Braganhol, E., de Freitas, V., Franzon, R. C., Reis, A. & Vizzotto, M. (2024). Food Science and Engineering, 300-321.
Psidium cattleianum Sabine (araçá) is a species native to Southeast Brazil that grows under abiotic stress conditions conferring high content of bioactive compounds to its fruits. The presence of these compounds is thought to be responsible for the many health-promoting effects including antioxidant, anti-inflammatory, anti-aging and antidiabetic activities. In this study, we evaluated the inhibitory potential of 10 (red and yellow) araçá genotypes towards carbohydrate-hydrolyzing enzymes (CHEs) using cell-free (α-glucosidase, α-amylase) and cell-based assays (sucrase). Araçá extracts displayed stronger inhibition towards α-glucosidase than α-amylase, and only 3 inhibited sucrase activity. The high variability towards the in vitro inhibitory CHEs activity was reflected in the total phenolics content with values ranging between 38.9 and 117 mg/100 g. Of the thirty compounds identified by High-Performance Liquid Chromatography-Diode Array Detection-Electrospray Ionization-Tandem Mass Spectrometry (HPLC-DAD-ESIMS/MS), including caffeic acids (9), organic acids (3) ellagitannins (15) and flavonoids (3), ellagitannins were the most abundant class. Statistical analysis showed ellagitannins were the main discriminators to the CHEs inhibitory activity. In summary, by expanding the panel of red and yellow araçá varieties studied, our results show that not all araçá genotypes inhibit CHE as only YA-23, RA-29, and RA-87 inhibited all 3 CHE which were related to the presence of ellagitannins. Information on the araçá genotypes with greater CHE inhibitory activity allied with the health-promoting effects of ellagitannin-rich foods, can be used to scale-up commercially exploitable genotypes with the aim to develop araçá-containing food formulations targeted to the pre-diabetic population.
Hide AbstractEffects of the kinetic pattern of dietary glucose release on nitrogen utilization, the portal amino acid profile, and nutrient transporter expression in intestinal enterocytes in piglets.
Li, Z., Li, Y., Zhao, Y., Wang, G., Liu, R., Li, Y., Aftab, Q., Sun, Z. & Zhong, Q. (2024). Journal of Animal Science and Biotechnology, 15(1), 49.
Background: Promoting the synchronization of glucose and amino acid release in the digestive tract of pigs could effectively improve dietary nitrogen utilization. The rational allocation of dietary starch sources and the exploration of appropriate dietary glucose release kinetics may promote the dynamic balance of dietary glucose and amino acid supplies. However, research on the effects of diets with different glucose release kinetic profiles on amino acid absorption and portal amino acid appearance in piglets is limited. This study aimed to investigate the effects of the kinetic pattern of dietary glucose release on nitrogen utilization, the portal amino acid profile, and nutrient transporter expression in intestinal enterocytes in piglets. Methods: Sixty-four barrows (15.00 ± 1.12 kg) were randomly allotted to 4 groups and fed diets formulated with starch from corn, corn/barley, corn/sorghum, or corn/cassava combinations (diets were coded A, B, C, or D respectively). Protein retention, the concentrations of portal amino acid and glucose, and the relative expression of amino acid and glucose transporter mRNAs were investigated. In vitro digestion was used to compare the dietary glucose release profiles. Results: Four piglet diets with different glucose release kinetics were constructed by adjusting starch sources. The in vivo appearance dynamics of portal glucose were consistent with those of in vitro dietary glucose release kinetics. Total nitrogen excretion was reduced in the piglets in group B, while apparent nitrogen digestibility and nitrogen retention increased (P < 0.05). Regardless of the time (2 h or 4 h after morning feeding), the portal total free amino acids content and contents of some individual amino acids (Thr, Glu, Gly, Ala, and Ile) of the piglets in group B were significantly higher than those in groups A, C, and D (P < 0.05). Cluster analysis showed that different glucose release kinetic patterns resulted in different portal amino acid patterns in piglets, which decreased gradually with the extension of feeding time. The portal His/Phe, Pro/Glu, Leu/Val, Lys/Met, Tyr/Ile and Ala/Gly appeared higher similarity among the diet treatments. In the anterior jejunum, the glucose transporter SGLT1 was significantly positively correlated with the amino acid transporters B0AT1, EAAC1, and CAT1. Conclusions: Rational allocation of starch resources could regulate dietary glucose release kinetics. In the present study, group B (corn/barley) diet exhibited a better glucose release kinetic pattern than the other groups, which could affect the portal amino acid contents and patterns by regulating the expression of amino acid transporters in the small intestine, thereby promoting nitrogen deposition in the body, and improving the utilization efficiency of dietary nitrogen.
Hide AbstractThe Antibacterial Properties of Polish Honey against Streptococcus mutans—A Causative Agent of Dental Caries.
Grabek-Lejko, D. & Hyrchel, T. (2023). Antibiotics, 12(11), 1640.
Streptococcus mutans is considered the main pathogen responsible for dental caries, one of the major infectious diseases, affecting more than 4 billion people worldwide. Honey is a natural product with well-known antibacterial potential against several human pathogens. The aim of the study was to evaluate the antibacterial efficacy of Polish honey against S. mutans and analyze the role of some bioactive substances on its antibacterial action. The antibacterial potential of different honey varieties (goldenrod, buckwheat, honeydew, and lime) was analyzed using a microdilution assay. Manuka and artificial honey were used as controls. The content of GOX, hydrogen peroxide, total polyphenols, and antioxidant potential was assayed in honey. The influence of catalase and proteinase K on antibacterial activity as well as antibiofilm action was also determined. The strongest antibacterial activity was observed for buckwheat, honeydew, and manuka honey, which were also characterized by the highest antioxidant activity and polyphenols content. Catalase treatment decreases the antibacterial activity of honey, while proteinase K treatment influences the antibacterial potential of honey slightly less. Obtained results suggest that honey can be a good natural product against S. mutans, and hydrogen peroxide was identified as a crucial contributor to its antimicrobial action.
Hide AbstractCharacterisation of physicochemical parameters and antibacterial properties of New Caledonian honeys.
Bucekova, M., Godocikova, J., Gueyte, R., Chambrey, C. & Majtan, J. (2023). Plos one, 18(10), e0293730.
Honey is an attractive natural product with various health benefits. A few honey-based commercial products have successfully been adopted in clinics to improve wound healing. However, screening of other potential sources of medical-grade honey, in particular, honeys from territories with high floral species diversity and high endemicity, is highly needed. The goal of this study was to characterise the physicochemical and antibacterial properties of New Caledonian honey samples (n = 33) and to elucidate the major mechanism of their antibacterial action. Inhibitory antibacterial activity of honeys against Staphylococcus aureus and Pseudomonas aeruginosa was determined with a minimum inhibitory concentration (MIC) assay. Enzymatic activity of glucose oxidase and the content of hydrogen peroxide (H2O2) in honey samples were analysed. Furthermore, total protein content of honeys together with their electrophoretic protein profiles were also determined in the study. The antibacterial efficacy of 24% of the tested honey samples was slightly superior to that of manuka honey with unique manuka factor 15+. The antibacterial activity of catalase-treated honey sample solutions was significantly reduced, suggesting that H2O2 is a key antibacterial compound of diluted honeys. However, the kinetic profiles of H2O2 production in most potent honeys at a MIC value of 6% was not uniform. Under the experimental conditions, we found that a H2O2 concentration of 150 μM in diluted honeys is a critical concentration for inhibiting the growth of S. aureus. In contrast, 150 μM H2O2 in artificial honey solution was not able to inhibit bacterial growth, suggesting a role of phytochemicals in the antibacterial activity of natural honey. In addition, the continuous generation of H2O2 in diluted honey demonstrated an ability to counteract additional bacteria in re-inoculation experiments. In conclusion, the tested New Caledonian honey samples showed strong antibacterial activity, primarily based on H2O2 action, and therefore represent a suitable source for medical-grade honey.
Hide AbstractPlant immunity suppression by an exo-β-1, 3-glucanase and an elongation factor 1α of the rice blast fungus.
Liu, H., Lu, X., Li, M., Lun, Z., Yan, X., Yin, C., .et al. (2023). Nature Communications, 14(1), 5491.
Fungal cell walls undergo continual remodeling that generates β-1,3-glucan fragments as products of endo-glycosyl hydrolases (GHs), which can be recognized as pathogen-associated molecular patterns (PAMPs) and trigger plant immune responses. How fungal pathogens suppress those responses is often poorly understood. Here, we study mechanisms underlying the suppression of β-1,3-glucan-triggered plant immunity by the blast fungus Magnaporthe oryzae. We show that an exo-β-1,3-glucanase of the GH17 family, named Ebg1, is important for fungal cell wall integrity and virulence of M. oryzae. Ebg1 can hydrolyze β-1,3-glucan and laminarin into glucose, thus suppressing β-1,3-glucan-triggered plant immunity. However, in addition, Ebg1 seems to act as a PAMP, independent of its hydrolase activity. This Ebg1-induced immunity appears to be dampened by the secretion of an elongation factor 1 alpha protein (EF1α), which interacts and co-localizes with Ebg1 in the apoplast. Future work is needed to understand the mechanisms behind Ebg1-induced immunity and its suppression by EF1α.
Hide AbstractEffects of glucose release kinetics of extruded-maize diet on energy utilization of growing pigs.
Zhu, H., Zhao, Y., Mi, M., Zhang, Q., Fu, X., Zheng, Y., Qin, G., Pan, L. & Bao, N. (2023). Animal Feed Science and Technology, 304, 115747.
The precise energy requirement of swine should not only meet the static parameters of net energy but also consider the dynamic supply of glucose release. The effects of glucose release characteristics on nitrogen and energy utilization of growing pigs were conducted by the energy metabolism in vivo using open-circuit respiratory calorimetry and the hydrolysis in vitro of glucose release. Sixteen castrated boars were randomly divided into two groups (Raw maize group and Extruded maize group, RAW and EXT). Maize in the RAW diet was completely replaced by extruded-maize in the EXT diet. In vitro studies showed that extrusion treatment had a rapid and huge glucose release of maize (P < 0.05). The metabolizable energy (ME) in EXT was higher than that in RAW (P = 0.002), but no difference in net energy (NE) was observed (P = 0.864). EXT had higher crude protein (CP) apparent digestibility (P = 0.035) but lower nitrogen deposition (P < 0.001) due to higher urinary nitrogen (UN) excretion (P < 0.001). Extrusion treatment changed the proportion of heat production of carbohydrates, fat, and protein. EXT presented higher oxidation of protein (OXP, P < 0.001) and fat (OXF, P = 0.025) and lower oxidation of carbohydrates (OXCHO, P = 0.003). There was no difference in retained energy (RE) between the two groups (P = 0.803). Extrusion treatment can accelerate the glucose release of maize, and the extruded maize fully replacing raw maize in the diet can enhance ME of growing pigs, and the ratio of nutrient oxidation to energy supply was altered, resulting in lower protein deposition and energy utilization.
Hide AbstractAntibacterial and enzyme inhibitory activities of flavan-3-ol monomers and procyanidin-rich grape seed fractions.
Ares, P. S., Gaur, G., Willing, B. P., Weber, F., Schieber, A. & Gänzle, M. G. (2023). Journal of Functional Foods, 107, 105643.
This study aimed to determine structure–function relationships of monomeric and oligomeric flavan-3-ols (procyanidins) with respect to their antimicrobial activity and their inhibition of digestive enzymes. Monomeric flacan-3-ols were purchased as reference compounds; oligomeric procyanidins were extracted from grape seeds, separated by high-performance counter-current chromatography and characterized by LC-MS/MS. The antimicrobial activity against a broad range of intestinal microorganisms increased in the order catechin = epicatechin > epigallocatechin and oligomeric procyanidins > epigallocatechin gallate. Facultative anaerobes were highly resistant while strict anaerobes including Allobaculum sp. and Ruminococcus gnavus were 10 – 100 times more sensitive. The inhibition of digestive enzymes from the rat small intestine increased in the order catechin < epicatechin and oligomeric procyanidins < epigallocatechin < epigallocatechin gallate. Trimeric and polymeric procyanidins were more inhibitory than dimeric procyanidins. Both the antimicrobial and enzyme-inhibitory activities of procyanidins and flavan-3-ols likely relate to their microbiome-modulating and health beneficial properties.
Hide AbstractDevelopment of phytoglycogen-derived core–shell–corona nanoparticles complexed with conjugated linoleic acid.
Wang, Z., Hu, X., Hamaker, B. R., Zhang, T. & Miao, M. (2023). Food & Function, In Press.
Phytoglycogen-derived self-assembled nanoparticles (SMPG/CLA) and enzymatic-assembled nanoparticles (EMPG/CLA) were fabricated for delivery of conjugated linoleic acid (CLA). After measuring the loading rate and yield, the optimal ratio for both assembled host–guest complexes was 1 : 10, and the maximum loading rate and yield for EMPG/CLA were 1.6% and 88.1%, respectively, higher than those of SMPG/CLA. Structural characterization studies showed that the assembled inclusion complexes were successfully constructed, and had a specific spatial architecture with inner-core amorphous and external-shell crystalline parts. A higher protective effect against oxidation of EMPG/CLA was observed than that of SMPG/CLA, supporting efficient complexation for a higher order crystalline structure. After 1 h of gastrointestinal digestion under the simulated conditions, 58.7% of CLA was released from EMPG/CLA, which was lower than that released from SMPG/CLA (73.8%). These results indicated that in situ enzymatic-assembled phytoglycogen-derived nanoparticles might be a promising carrier platform for protection and targeted delivery of hydrophobic bioactive ingredients.
Hide AbstractPhysicochemical properties and in vitro digestibility of resistant starches obtained by autoclaving and lintnerization from native corn, apple and malanga starches.
Aguilar-Mendoza, V., Zamudio-Flores, P. B., Molina-Corral, F. J., Olivas-Orozco, G. I., Vela-Gutierrez, G., Hernandez-Gonzalez, M., et al. (2023). Biotecnia, 25(2), 12-22.
In this research, the effect of the application of successive autoclaving/cooling and lintnerization (acid hydrolysis) cycles in native starches of corn (NCS), apple (NAS), and malanga (NMS) on the formation of resistant starch (RS) was evaluated, and the physicochemical properties and in vitro digestibility of the obtained starches were studied. Autoclaved malanga starch (AMS) presented the highest RS content (14 %) compared to all analyzed starches. Enzymatic hydrolysis of native and modified starches indicated that autoclaving/ cooling decreased amylolysis (≈ 24 - 41 %), while with the lintnerization treatment, the reduction was lower (≈ 3 - 21 %), both results compared to their native counterparts. Autoclaving and lintnerization treatments reduced the apparent amylose content by ≈ 5 %, producing amylose with lower molecular weights (≈ 80 - 87 kDa) for autoclaved starches, and ≈ 92 - 101 kDa for lintnerized starches. The luminosity decreased due to the autoclaving treatment and not to the lintnerization process. These results suggest that malanga starch subjected to autoclaving/cooling cycles could be used in baking applications where an increase of the RS content without affecting the sensory color characteristics is desired.
Hide AbstractProduction of docosahexaenoic acid from spruce sugars using Aurantiochytrium limacinum.
Olsen, P. M., Kósa, G., Klüver, M., Kohler, A., Shapaval, V. & Horn, S. J. (2023). Bioresource Technology, 376, 128827.
In this study lignocellulosic sugars from Norway spruce were used for production of docosahexaenoic acid (DHA) by the marine thraustochytrid Aurantiochytrium limacinum SR21. Enzymatically prepared spruce hydrolysate was combined with a complex nitrogen source and different amounts of salts. Shake flask batch cultivations revealed that addition of extra salts was not needed for optimal growth. Upscaling to fed-batch bioreactors yielded up to 55 g/L cell dry mass and a total fatty acid content of 44% (w/w) out of which 1/3 was DHA. Fourier transform infrared spectroscopy was successfully applied as a rapid method for monitoring lipid accumulation in A. limacinum SR21. Thus, this proof-of-principle study clearly demonstrates that crude spruce hydrolysates can be directly used as a novel and sustainable resource for production of DHA.
Hide Abstract