| Content: | 1,200 Units |
| Shipping Temperature: | Ambient |
| Storage Temperature: | 4oC |
| Formulation: | In 3.2 M ammonium sulphate |
| Physical Form: | Suspension |
| Stability: | > 4 years at 4oC |
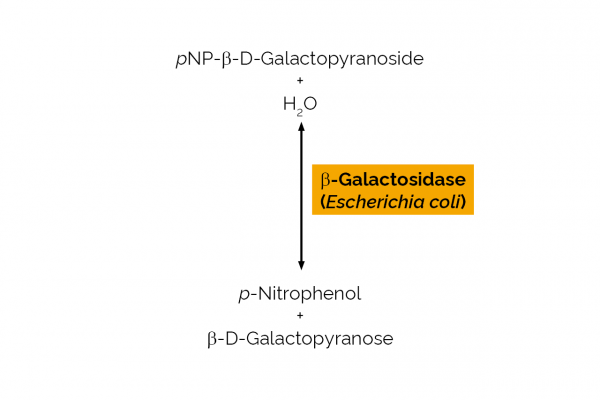
| Enzyme Activity: | β-Galactosidase |
| EC Number: | 3.2.1.23 |
| CAZy Family: | GH2 |
| CAS Number: | 9031-11-2 |
| Synonyms: | beta-D-galactoside galactohydrolase |
| Source: | Escherichia coli |
| Molecular Weight: | 116,000 |
| Concentration: | Supplied at ~ 900 U/mL |
| Expression: | From Escherichia coli |
| Specificity: | Hydrolysis of terminal non-reducing β-D-galactose residues in β-D-galactosides. |
| Specific Activity: | ~ 35 U/mg (40oC, pH 6.5 on p-nitrophenyl-β-D-galactoside) |
| Unit Definition: | One Unit of β-galactosidase activity is defined as the amount of enzyme required to release one µmole of p-nitrophenol per minute from p-nitrophenyl-β-D-galactoside (10 mM) in sodium phosphate buffer (100 mM), pH 6.5 at 40oC. |
| Temperature Optima: | 40oC |
| pH Optima: | 6.5 |
| Application examples: | For use in research. |
This product has been discontinued (read more).
High purity β-galactosidase (Escherichia coli) for use in research, biochemical enzyme assays and in vitro diagnostic analysis.
Browse our complete CAZy enzyme products range.
A novel enzymatic method for the measurement of lactose in lactose‐free products.
Mangan, D., McCleary, B. V., Culleton, H., Cornaggia, C., Ivory, R., McKie, V. A., Delaney, E. & Kargelis, T. (2018). Journal of the Science of Food and Agriculture, 99, 947-956.
Background: In recent years there has been a surge in the number of commercially available lactose‐free variants of a wide variety of products. This presents an analytical challenge for the measurement of the residual lactose content in the presence of high levels of mono‐, di‐, and oligosaccharides. Results: In the current work, we describe the development of a novel enzymatic low‐lactose determination method termed LOLAC (low lactose), which is based on an optimized glucose removal pre‐treatment step followed by a sequential enzymatic assay that measures residual glucose and lactose in a single cuvette. Sensitivity was improved over existing enzymatic lactose assays through the extension of the typical glucose detection biochemical pathway to amplify the signal response. Selectivity for lactose in the presence of structurally similar oligosaccharides was provided by using a β-galactosidase with much improved selectivity over the analytical industry standards from Aspergillus oryzae and Escherichia coli (EcLacZ), coupled with a ‘creep’ calculation adjustment to account for any overestimation. The resulting enzymatic method was fully characterized in terms of its linear range (2.3-113 mg per 100 g), limit of detection (LOD) (0.13 mg per 100 g), limit of quantification (LOQ) (0.44 mg per 100 g) and reproducibility (≤ 3.2% coefficient of variation (CV)). A range of commercially available lactose‐free samples were analyzed with spiking experiments and excellent recoveries were obtained. Lactose quantitation in lactose‐free infant formula, a particularly challenging matrix, was carried out using the LOLAC method and the results compared favorably with those obtained from a United Kingdom Accreditation Service (UKAS) accredited laboratory employing quantitative high performance anion exchange chromatography - pulsed amperometric detection (HPAEC‐PAD) analysis. Conclusion: The LOLAC assay is the first reported enzymatic method that accurately quantitates lactose in lactose‐free samples.
Hide Abstract